| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347029 | 980291 | 2009 | 9 صفحه PDF | دانلود رایگان |
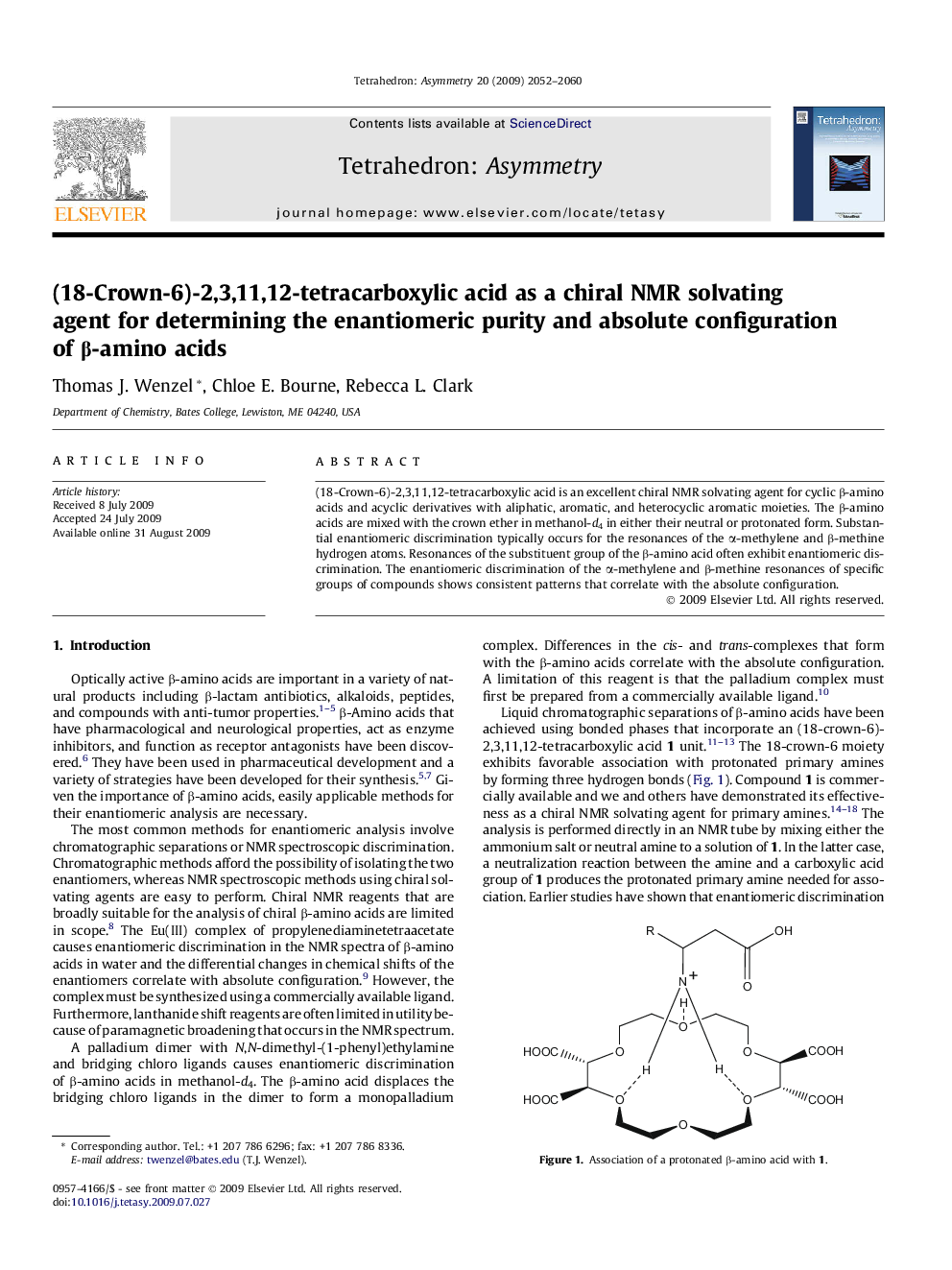
(18-Crown-6)-2,3,11,12-tetracarboxylic acid is an excellent chiral NMR solvating agent for cyclic β-amino acids and acyclic derivatives with aliphatic, aromatic, and heterocyclic aromatic moieties. The β-amino acids are mixed with the crown ether in methanol-d4 in either their neutral or protonated form. Substantial enantiomeric discrimination typically occurs for the resonances of the α-methylene and β-methine hydrogen atoms. Resonances of the substituent group of the β-amino acid often exhibit enantiomeric discrimination. The enantiomeric discrimination of the α-methylene and β-methine resonances of specific groups of compounds shows consistent patterns that correlate with the absolute configuration.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 17, 8 September 2009, Pages 2052–2060