| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347031 | 980291 | 2009 | 7 صفحه PDF | دانلود رایگان |
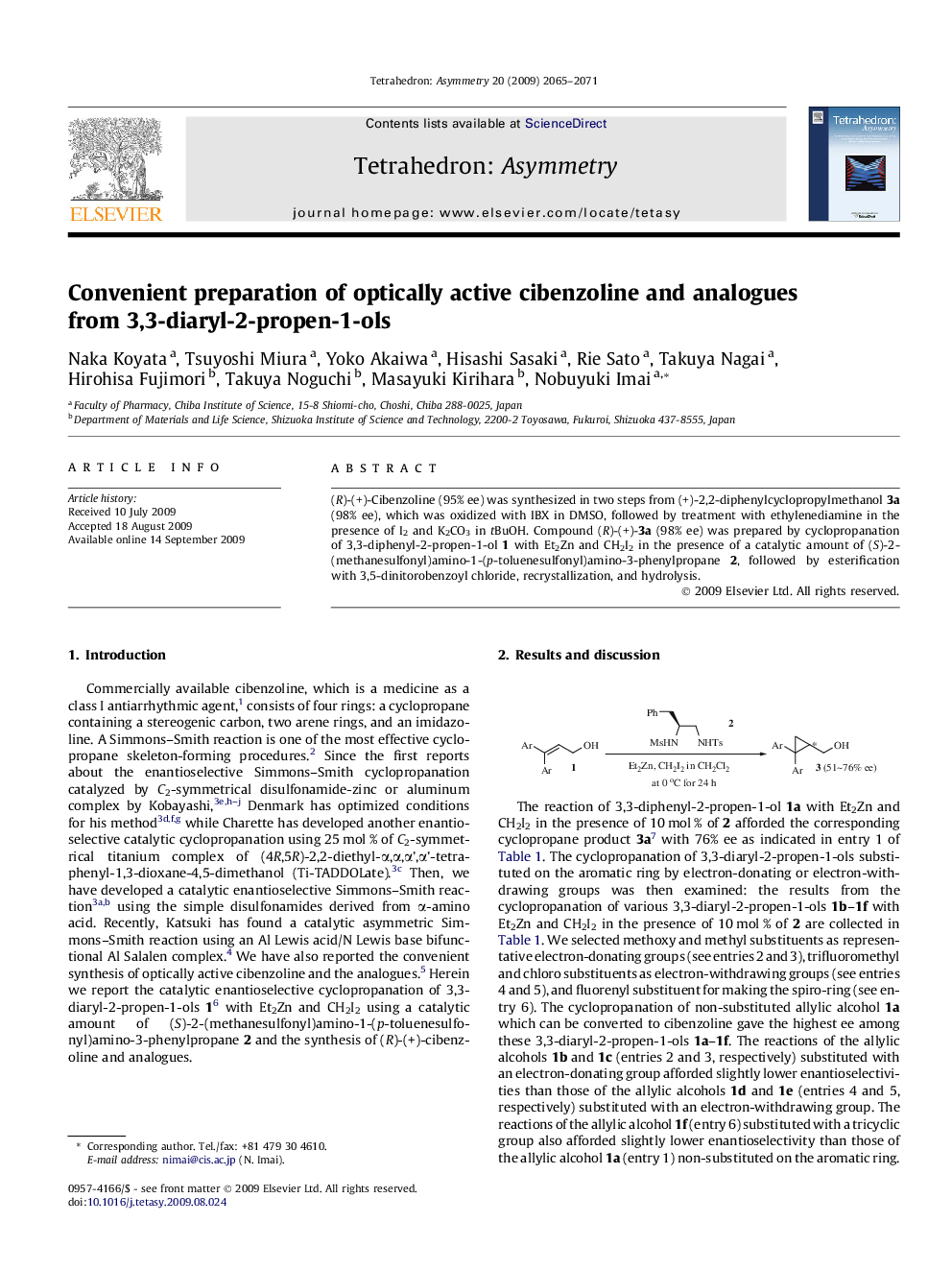
(R)-(+)-Cibenzoline (95% ee) was synthesized in two steps from (+)-2,2-diphenylcyclopropylmethanol 3a (98% ee), which was oxidized with IBX in DMSO, followed by treatment with ethylenediamine in the presence of I2 and K2CO3 in tBuOH. Compound (R)-(+)-3a (98% ee) was prepared by cyclopropanation of 3,3-diphenyl-2-propen-1-ol 1 with Et2Zn and CH2I2 in the presence of a catalytic amount of (S)-2-(methanesulfonyl)amino-1-(p-toluenesulfonyl)amino-3-phenylpropane 2, followed by esterification with 3,5-dinitorobenzoyl chloride, recrystallization, and hydrolysis.
Figure optionsDownload as PowerPoint slide
(R)-2-(2,2-Diphenylcyclopropyl)imidazolineC18H18N2Ee = 72%[α]D24=+110.0 (c 1.12, MeOH)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: (R)
(R)-2,2-DiphenylcyclopropanecarboxaldehydeC16H14OEe = 76%[α]D24=+112.4 (c 1.47, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: (R)
(R)-2,2-DiphenylcyclopropylmethanolC16H16OEe = 76%[α]D24=+115.9 (c 1.07, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: (R)
2,2-Bis(4-methoxyphenyl)cyclopropylmethanolC18H20O3Ee = 51%[α]D24=+74.2 (c 1.04, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2-(2,2-Bis(4-methylphenyl)cyclopropyl)imidazolineC20H22N2Ee = 65%[α]D23=+66.0 (c 0.90, MeOH)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-Bis(4-methylphenyl)cyclopropanecarboxaldehydeC18H18OEe = 65%[α]D27=+77.9 (c 1.37, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-Bis(4-methylphenyl)cyclopropylmethanolC18H20OEe = 65%[α]D24=+101.7 (c 1.45, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2-(2,2-Bis(4-trifluoromethylphenyl)cyclopropyl)imidazolineC20H16F6N2Ee = 74%[α]D23=+68.6 (c 1.18, MeOH)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-Bis(4-trifluoromethylphenyl)cyclopropanecarboxaldehydeC18H12F6OEe = 73%[α]D27=+86.4 (c 1.43, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-Bis(4-trifluoromethylphenyl)cyclopropylmethanolC18H14F6OEe = 73%[α]D24=+80.0 (c 1.41, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2-(2,2-Bis(4-chlorophenyl)cyclopropyl)imidazolineC18H16Cl2N2Ee = 71%[α]D24=+86.0 (c 0.97, MeOH)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-Bis(4-chlorophenyl)cyclopropanecarboxaldehydeC16H12Cl2OEe = 72%[α]D27=+95.0 (c 0.65, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-Bis(4-chlorophenyl)cyclopropylmethanolC16H14Cl2OEe = 72%[α]D24=+100.4 (c 0.78, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2-(2,2-Fluorenylcyclopropyl)imidazolineC18H16N2Ee = 58%[α]D27=+186.4 (c 0.34, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-FluorenylcyclopropanecarboxaldehydeC16H12OEe = 60%[α]D27=+125.5 (c 0.99, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
2,2-FluorenylcyclopropylmethanolC16H14OEe = 60%[α]D24=+12.5 (c 1.12, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: unknown
(1S,2S)-2-Methyl-2-phenylcyclopropylmethanolC11H14OEe = 38%[α]D24=+24.1 (c 1.01, CHCl3)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: (1S,2S)
(4R,5R,1′R)-2-(2,2-Diphenylcyclopropyl)-4,5-diphenyl-2-imidazolineC30H26N2Ee = 98%[α]D27=+152.5 (c 1.00, MeOH)Source of chirality: catalytic enantioselective cyclopropanationAbsolute configuration: (4R,5R,1′R)
(4R,5R,1′R)-2-(2,2-Diphenylcyclopropyl)-4,5-cyclohexanimidazolineC22H24N2
• Ee = 98%
• [α]D27=+177.1 (c 0.71, MeOH)
• Source of chirality: catalytic enantioselective cyclopropanation
• Absolute configuration: (4R,5R,1′R)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 17, 8 September 2009, Pages 2065–2071