| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347041 | 980292 | 2014 | 7 صفحه PDF | دانلود رایگان |
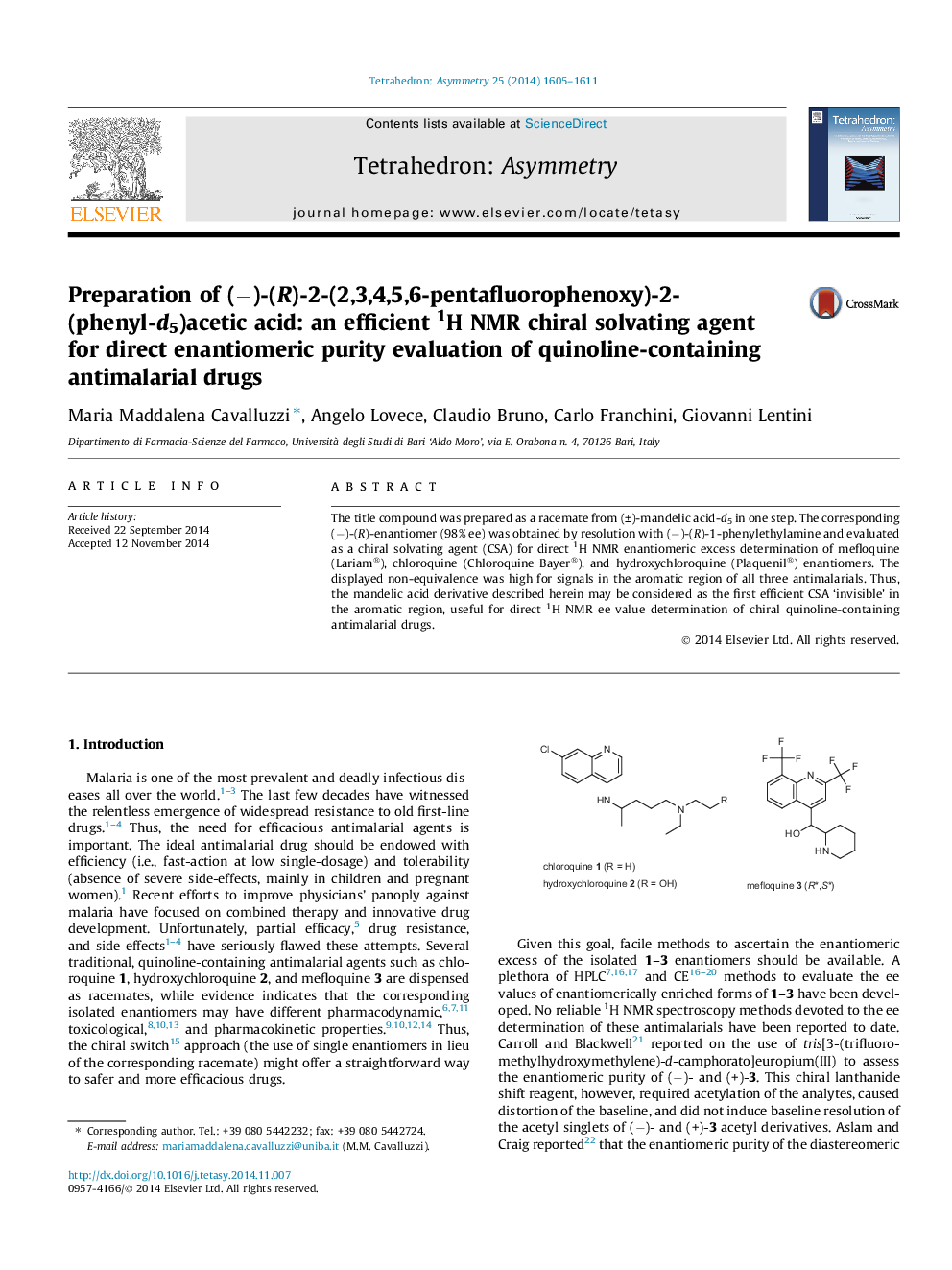
The title compound was prepared as a racemate from (±)-mandelic acid-d5 in one step. The corresponding (−)-(R)-enantiomer (98% ee) was obtained by resolution with (−)-(R)-1-phenylethylamine and evaluated as a chiral solvating agent (CSA) for direct 1H NMR enantiomeric excess determination of mefloquine (Lariam®), chloroquine (Chloroquine Bayer®), and hydroxychloroquine (Plaquenil®) enantiomers. The displayed non-equivalence was high for signals in the aromatic region of all three antimalarials. Thus, the mandelic acid derivative described herein may be considered as the first efficient CSA ‘invisible’ in the aromatic region, useful for direct 1H NMR ee value determination of chiral quinoline-containing antimalarial drugs.
Figure optionsDownload as PowerPoint slide
(−)-(R)-2-(2,3,4,5,6-Pentafluorophenoxy)-2-(phenyl-d5)acetic acidC14H2D5F5O3Ee = 98%[α]D20 = −151 (c 2, CHCl3)Source of chirality: resolutionAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 25, Issue 24, 31 December 2014, Pages 1605–1611