| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347045 | 980292 | 2014 | 13 صفحه PDF | دانلود رایگان |
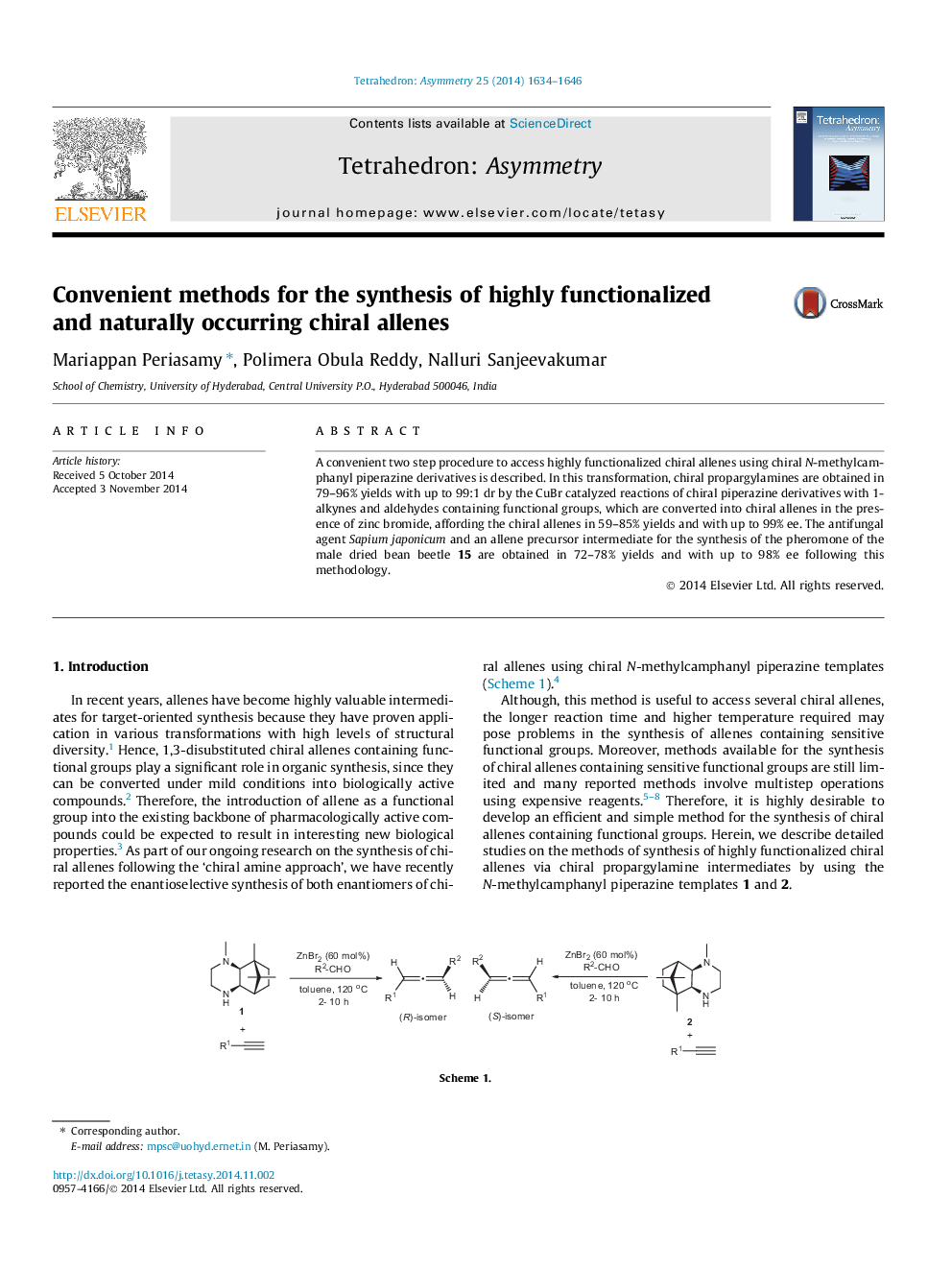
A convenient two step procedure to access highly functionalized chiral allenes using chiral N-methylcamphanyl piperazine derivatives is described. In this transformation, chiral propargylamines are obtained in 79–96% yields with up to 99:1 dr by the CuBr catalyzed reactions of chiral piperazine derivatives with 1-alkynes and aldehydes containing functional groups, which are converted into chiral allenes in the presence of zinc bromide, affording the chiral allenes in 59–85% yields and with up to 99% ee. The antifungal agent Sapium japonicum and an allene precursor intermediate for the synthesis of the pheromone of the male dried bean beetle 15 are obtained in 72–78% yields and with up to 98% ee following this methodology.
Figure optionsDownload as PowerPoint slide
7-Phenyl-7-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hept-5-ynoic acid ethyl esterC27H38N2O2dr = 99:1[α]D25 = −51.1 (c 0.43, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-[5,5-Dimethyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-2-methyl-malonic acid diethyl esterC29H48N2O4dr = 97:3[α]D25 = −52.0 (c 0.35, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-[5,5-Dimethyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-malonic acid dimethyl esterC26H42N2O4dr = 96:4[α]D25 = −43.6 (c 0.40, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-Methyl-2-[4-phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-ynyl]-malonic acid dimethyl esterC29H40N2O4dr = 98:2[α]D25 = −50.7 (c 0.52, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-Methyl-2-[6-phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-malonic acid dimethyl esterC31H44N2O4dr = 97:3[α]D25 = −48.1 (c 0.60, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-[5,5-Dimethyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-2-methyl-malonic acid dimethyl esterC27H44N2O4dr = 96:4[α]D25 = −39.7 (c 0.32, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-[4-Cyclohexyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-ynyl]-2-methyl-malonic acid dimethyl esterC29H46N2O4dr = 99:1[α]D25 = −36.9 (c 0.54, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-Benzyl-2-[5,5-dimethyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-malonic acid dimethyl esterC33H48N2O4dr = 96:4[α]D25 = −39.7 (c 2.5, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-Acetylamino-2-[4-phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-ynyl]-malonic acid dimethyl esterC32H45N3O5dr = 99:1[α]D25 = −42.1 (c 0.53, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-Acetylamino-2-[5,5-dimethyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-malonic acid dimethyl esterC30H49N3O5dr = 96:4[α]D25 = −36.5 (c 0.45, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
4-N-Dimethyl-N-[4-phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-ynyl]-benzenesulfonamideC31H41N3O2Sdr = 99:1[α]D25 = −54.2 (c 0.45, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
N-[5,5-Dimethyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-4,N-dimethyl-benzenesulfonamideC29H45N3O2Sdr = 98:2[α]D25 = −40.8 (c 0.40, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
N-Benzyl-4-methyl-N-[4-phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-ynyl]-benzenesulfonamideC37H45N3O2Sdr = 98:2[α]D25 = −40.8 (c 0.40, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
4-Chloro-N-[5,5-dimethyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-hex-2-ynyl]-N-methyl-benzenesulfonamideC28H42ClN3O2Sdr = 98:2[α]D25 = −31.7(c 0.40, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
(4-Phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-yn-1-olC24H34N2Odr = 99:1[α]D25 = −49.3 (c 0.75, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
(4-(4-Fluoro-phenyl)-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-yn-1-olC24H33FN2Odr = 96:4[α]D25 = −29.9 (c 0.80, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
1-[3-Cyclohexyl-3-(5,9,9-trimethyl-octahydro-5,8-methano-quinazolin-1-yl)-prop-1-ynyl)-cyclohexanolC28H46N2Odr = 95:5[α]D25 = −49.3 (c 0.80, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
8-(tert-Butyl-dimethyl-silanyloxy)-5-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-oct-6-ynoic acid methyl esterC28H50N2O3Sidr = 99:1[α]D25 = −37.4 (c 0.46, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
4-(4,5,9,9-Tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-tetradec-5-ynoic acid methyl esterC28H48N2O2dr = 99:1[α]D25 = −45.5 (c 0.62, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-Methyl-2-[4-phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-ynyl)-malonic acid dimethyl esterC29H40N2O4dr = 99:1[α]D25 = +43.6 (c 0.31, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
2-Acetylamino-2-[4-phenyl-4-(4,5,9,9-tetramethyl-octahydro-5,8-methano-quinazolin-1-yl)-but-2-ynyl]-malonic acid dimethyl esterC32H45N3O5dr = 99:1[α]D25 = +39.1 (c 0.23, CHCl3)Source of chirality: Diastereoselective synthesisAbsolute configuration: (4aS,5R,8S,8aR)
Journal: Tetrahedron: Asymmetry - Volume 25, Issue 24, 31 December 2014, Pages 1634–1646