| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347103 | 980294 | 2010 | 4 صفحه PDF | دانلود رایگان |
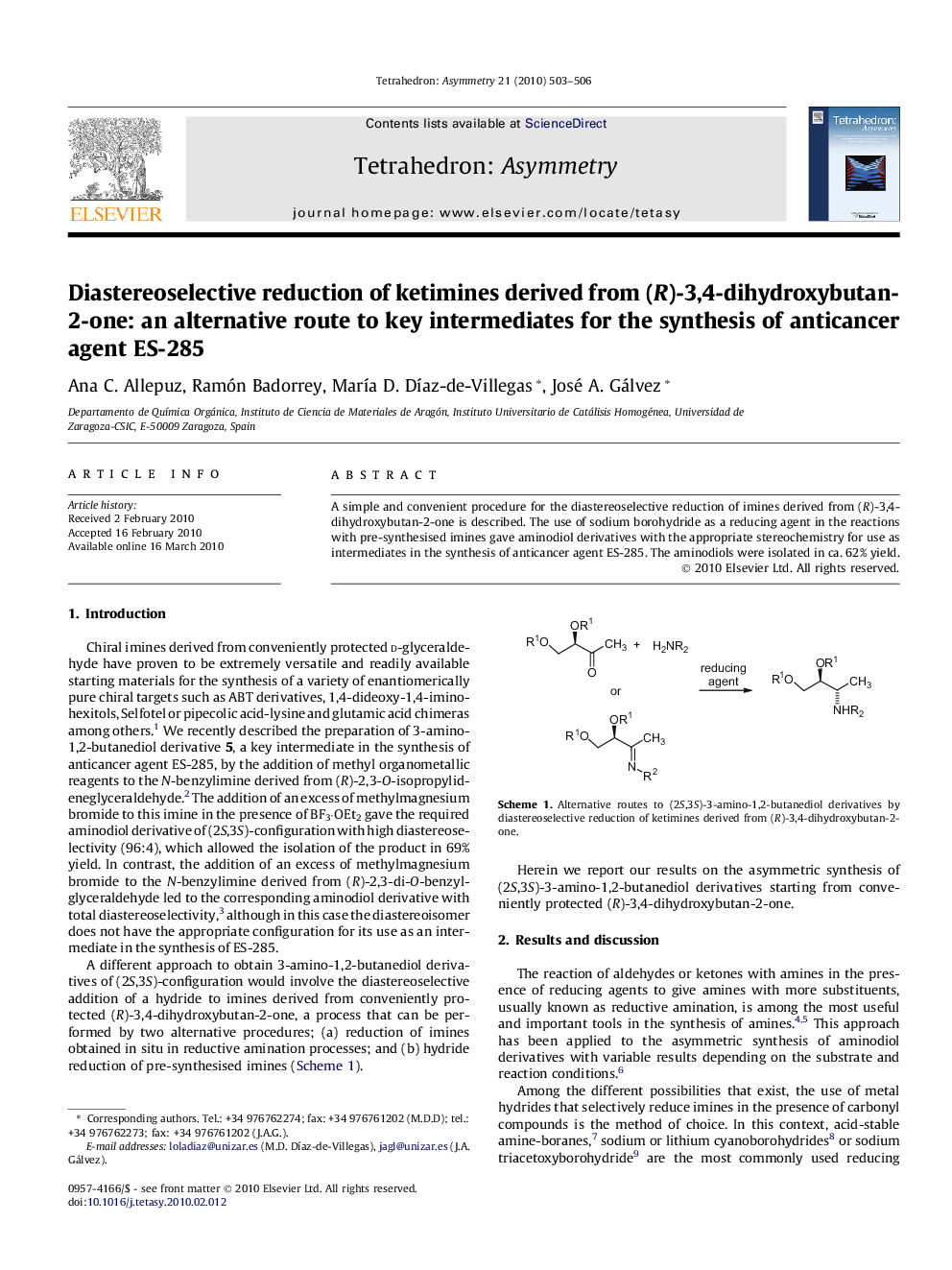
A simple and convenient procedure for the diastereoselective reduction of imines derived from (R)-3,4-dihydroxybutan-2-one is described. The use of sodium borohydride as a reducing agent in the reactions with pre-synthesised imines gave aminodiol derivatives with the appropriate stereochemistry for use as intermediates in the synthesis of anticancer agent ES-285. The aminodiols were isolated in ca. 62% yield.
Figure optionsDownload as PowerPoint slide
(R)-3,4-Bis(benzyloxy)butan-2-oneC18H20O3[α]D25=+32.2 (c 0.91, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (R)
(S)-N-Benzyl-1-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]ethanamineC14H21O2[α]D25=+37.1 (c 1.00, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (1S,1′S)
(2S,3S)-N-Benzyl-3,4-bis(benzyloxy)butan-2-amineC25H29NO2[α]D25=-3.5 (c 0.70, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (2S,3S)
Journal: Tetrahedron: Asymmetry - Volume 21, Issue 4, 16 March 2010, Pages 503–506