| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347121 | 1500343 | 2014 | 8 صفحه PDF | دانلود رایگان |
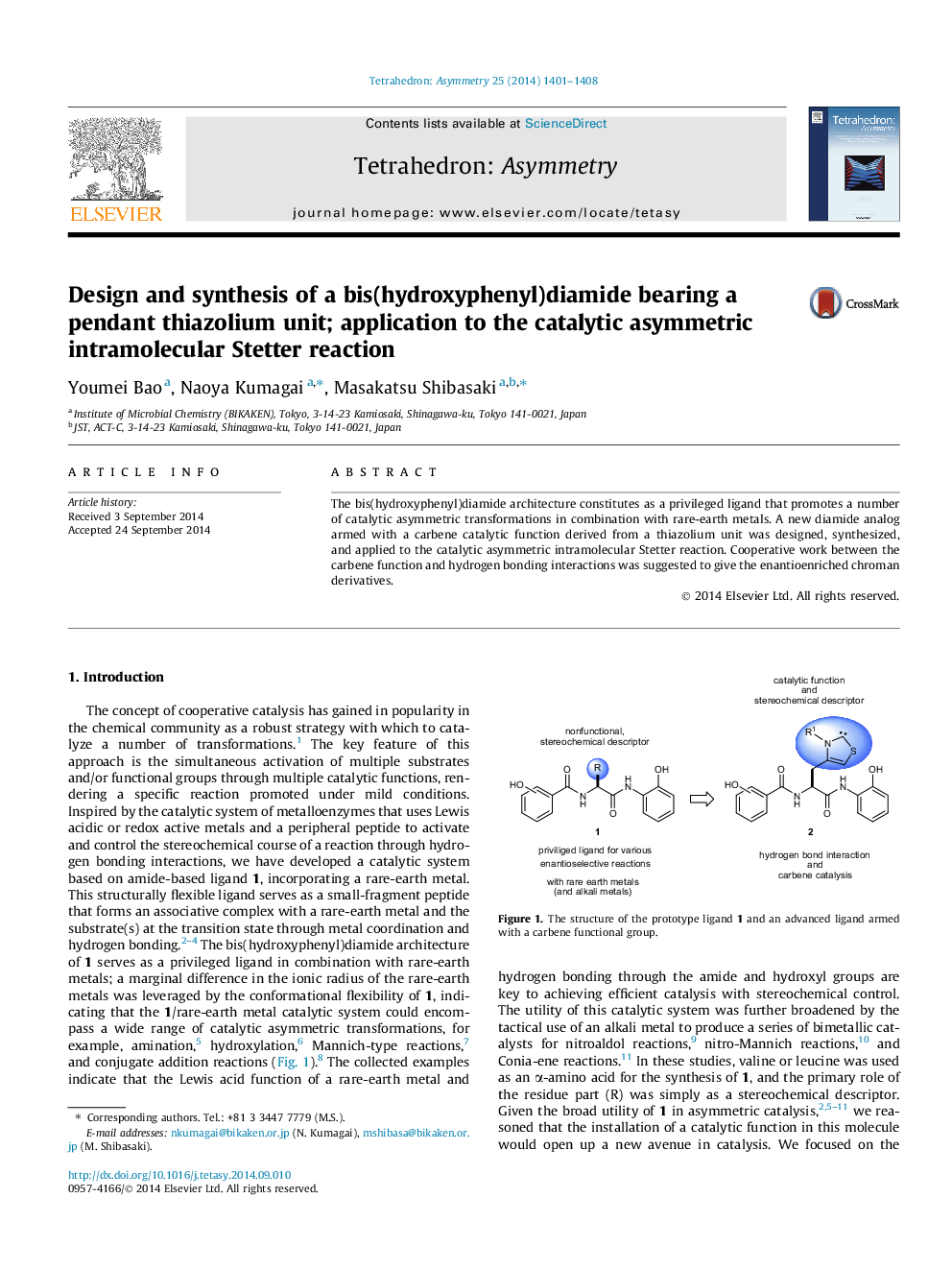
The bis(hydroxyphenyl)diamide architecture constitutes as a privileged ligand that promotes a number of catalytic asymmetric transformations in combination with rare-earth metals. A new diamide analog armed with a carbene catalytic function derived from a thiazolium unit was designed, synthesized, and applied to the catalytic asymmetric intramolecular Stetter reaction. Cooperative work between the carbene function and hydrogen bonding interactions was suggested to give the enantioenriched chroman derivatives.
Figure optionsDownload as PowerPoint slide
(R)-tert-Butyl 2-(4-oxochroman-3-yl)acetateC15H18O462% ee[α]D27 = −2.3 (c 1.8, CHCl3)Absolute configuration: (R)Source of chirality: Asymmetric synthesis
(R)-Ethyl 2-(4-oxochroman-3-yl)acetateC13H14O450% ee[α]D27 = −2.5 (c 1.7, CHCl3)Absolute configuration: (R)Source of chirality: Asymmetric synthesis
(R)-tert-Butyl 2-(6-methyl-4-oxochroman-3-yl)acetateC16H20O463% ee[α]D27 = −6.6 (c 1.7, CHCl3)Absolute configuration: (R)Source of chirality: Asymmetric synthesis
(R)-tert-Butyl 2-(6-methoxy-4-oxochroman-3-yl)acetateC16H20O559% ee[α]D27 = −12.7 (c 2.3, CHCl3)Absolute configuration: (R)Source of chirality: Asymmetric synthesis
(R)-tert-Butyl 2-(6-chloro-4-oxochroman-3-yl)acetateC15H17ClO459% ee[α]D27 = −0.4 (c 1.5, CHCl3)Absolute configuration: (R)Source of chirality: Asymmetric synthesis
(R)-Ethyl 3-(2-(tert-butoxy)-2-oxoethyl)-4-oxochroman-6-carboxylateC18H22O655% ee[α]D27 = −5.6 (c 1.9, CHCl3)Absolute configuration: (R)Source of chirality: Asymmetric synthesis
(S)-tert-Butyl (1-((2-(benzyloxy)phenyl)amino)-1-oxo-3-(thiazol-4-yl)propan-2-yl)carbamateC24H27N3O4S99% ee[α]D27 = −6.6 (c 0.7, CHCl3)Absolute configuration: (S)Source of chirality: l-Thiazolylalanine
(S)-2-(Benzyloxy)-N-(1-((2-(benzyloxy)phenyl)amino)-1-oxo-3-(thiazol-4-yl)propan-2-yl)benzamideC33H29N3O4S99% ee[α]D27 = +48.7 (c 0.5, CHCl3)Absolute configuration: (S)Source of chirality: l-Thiazolylalanine
(S)-4-(2-(Hydroxybenzamido)-3-((2-hydroxyphenyl)amino)-3-oxopropyl)-3-methylthiazol-3-ium iodideC20H20IN3O4S99% ee[α]D27 = −22.8 (c 0.5, CH3OH)Absolute configuration: (S)Source of chirality: l-Thiazolylalanine
(S)-3-Benzyl-4-(2-(2-hydroxybenzamido)-3-((2-hydroxyphenyl)amino)-3-oxopropyl)thiazol-3-ium bromideC26H24BrN3O4S99% ee[α]D27 = −26.4 (c 0.7, CH3OH)Absolute configuration: (S)Source of chirality: l-Thiazolylalanine
(S)-3-(4-(Decyloxy)benzyl)-4-(2-(2-hydroxybenzamido)-3-((2-hydroxyphenyl)amino)-3-oxopropyl)thiazol-3-ium bromideC36H44BrN3O5S99% ee[α]D27 = −17.8 (c 0.5, CHCl3)Absolute configuration: (S)Source of chirality: l-Thiazolylalanine
Journal: Tetrahedron: Asymmetry - Volume 25, Issues 20–21, 31 October 2014, Pages 1401–1408