| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347214 | 980300 | 2009 | 6 صفحه PDF | دانلود رایگان |
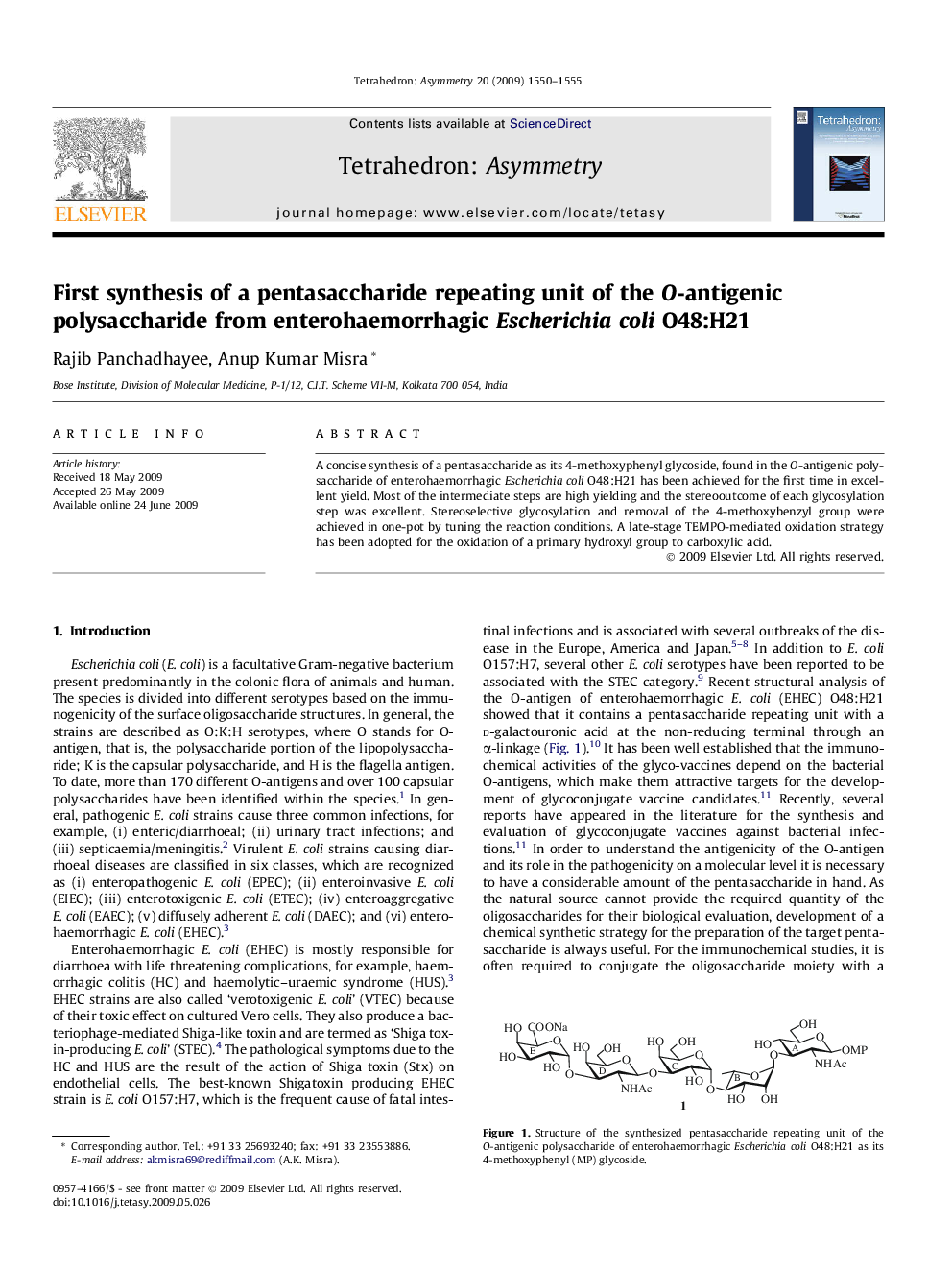
A concise synthesis of a pentasaccharide as its 4-methoxyphenyl glycoside, found in the O-antigenic polysaccharide of enterohaemorrhagic Escherichia coli O48:H21 has been achieved for the first time in excellent yield. Most of the intermediate steps are high yielding and the stereooutcome of each glycosylation step was excellent. Stereoselective glycosylation and removal of the 4-methoxybenzyl group were achieved in one-pot by tuning the reaction conditions. A late-stage TEMPO-mediated oxidation strategy has been adopted for the oxidation of a primary hydroxyl group to carboxylic acid.
Figure optionsDownload as PowerPoint slide
4-Methoxyphenyl (2,3-di-O-acetyl-4-O-allyl-α-l-rhamnopyranosyl)-(1→3)-4,6-O-benzylidene-2-deoxy-2-N-phthalimido-β-d-glucopyranosideC41H43NO14[α]D25=+9.3 (c 1.2, CHCl3)Source of chirality: l-rhamnose, d-glucosamine hydrochloride
4-Methoxyphenyl (2-O-benzyl-4,6-O-benzylidene-α-d-galactopyranosyl)-(1→4)-(2,3-di-O-acetyl-α-l-rhamnopyranosyl)-(1→3)-4,6-O-benzylidene-2-deoxy-2-N-phthalimido-β-d-glucopyranosideC58H59NO19[α]D25=-4 (c 1.2, CHCl3)Source of chirality: d-galactose, l-rhamnose, d-glucosamine hydrochloride
Ethyl (6-O-acetyl-2,3,4-tri-O-benzyl-α-d-galactopyranosyl)-(1→3)-4,6-O-benzylidene-2-deoxy-2-N-phthalimido-1-thio-β-d-galactopyranosideC52H53NO12S[α]D25=+81 (c 1.5, CHCl3)Source of chirality: d-galactose, d-glucosamine hydrochloride
4-Methoxyphenyl (6-O-acetyl-2,3,4-tri-O-benzyl-α-d-galactopyranosyl)-(1→3)-(4,6-O-benzylidene-2-deoxy-2-N-phthalimido-β-d-galactopyranosyl)-(1→3)-(2-O-benzyl-4,6-O-benzylidene-α-d-galactopyranosyl)-(1→4)-(2,3-di-O-acetyl-α-l-rhamnopyranosyl)-(1→3)-4,6-O-benzylidene-2-deoxy-2-N-phthalimido-β-d-glucopyranosideC108H106N2O31[α]D25=+66 (c 1.2, CHCl3)Source of chirality: d-galactose, d-glucosamine, l-rhamnose, d-glucosamine hydrochloride
4-Methoxyphenyl (sodium α-d-galactopyranosyl uronate)-(1→3)-(2-acetamido-2-deoxy-β-d-galactopyranosyl)-(1→3)-(α-d-galactopyranosyl)-(1→4)-(α-l-rhamnopyranosyl)-(1→3)-2-acetamido-2-deoxy-β-d-glucopyranosideC41H61N2NaO27[α]D25=+77 (c 1.0, H2O)Source of chirality: d-galactose, d-glucosamine hydrochloride, l-rhamnose, d-glucosamine hydrochloride
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 13, 16 July 2009, Pages 1550–1555