| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347250 | 980302 | 2009 | 9 صفحه PDF | دانلود رایگان |
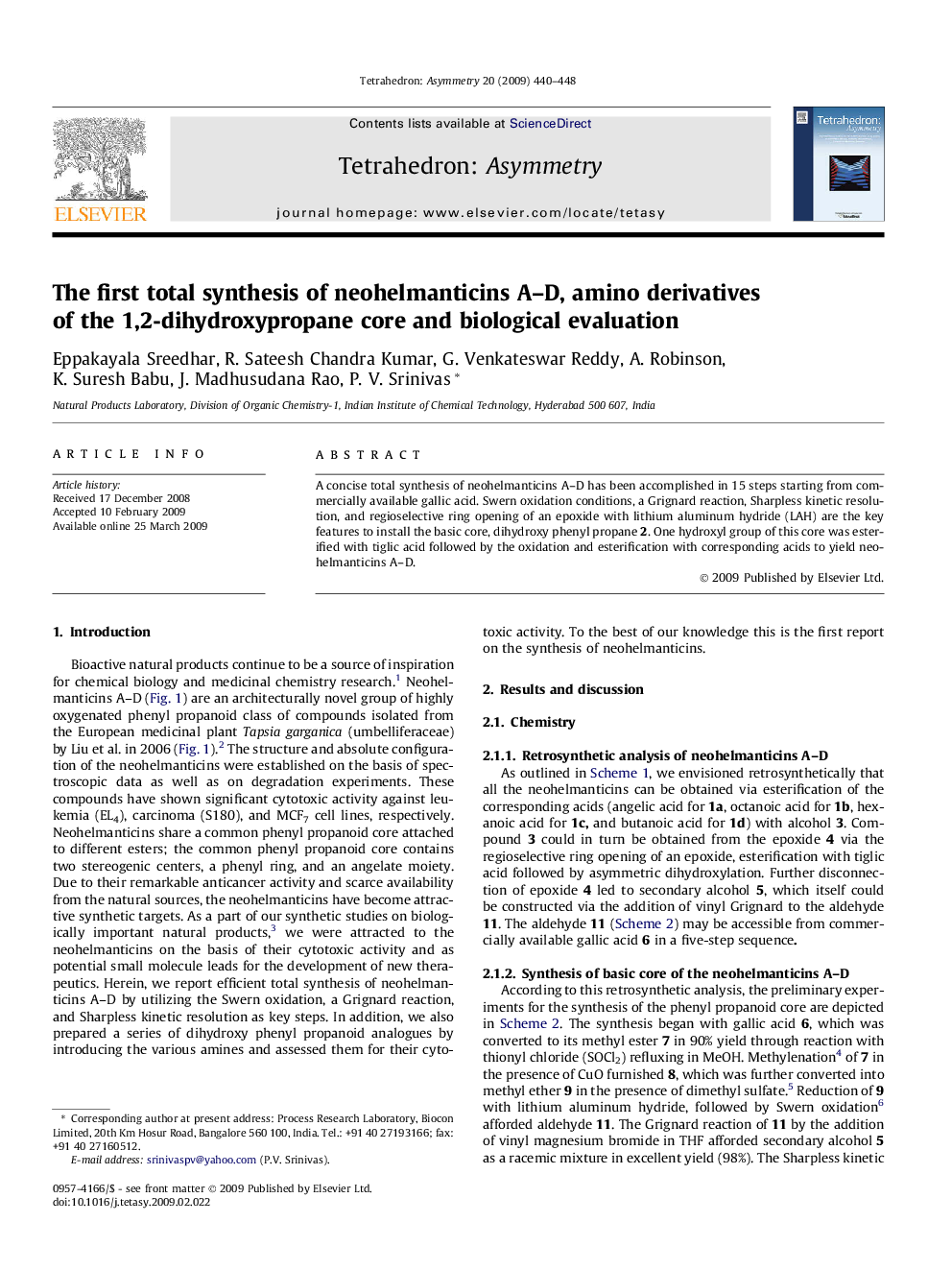
A concise total synthesis of neohelmanticins A–D has been accomplished in 15 steps starting from commercially available gallic acid. Swern oxidation conditions, a Grignard reaction, Sharpless kinetic resolution, and regioselective ring opening of an epoxide with lithium aluminum hydride (LAH) are the key features to install the basic core, dihydroxy phenyl propane 2. One hydroxyl group of this core was esterified with tiglic acid followed by the oxidation and esterification with corresponding acids to yield neohelmanticins A–D.
An efficient synthesis of natural products, neohelmanticins A–D, has been achieved in 15 steps starting from commercially available gallic acid.Figure optionsDownload as PowerPoint slide
(Z)-(2S,3R)-3-(((1R,2S)-1-Hydroxy-1-(4-methoxybenzo[d][1,3]dioxol-6-yl)propan-2-yloxy)carbonyl)-3-hydroxybutan-2-yl 2-methylbut-2-enoateC21H28O9[α]D = −23.1 (c 1.5, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,3R,1′R,2′S)
(2S,3R)-3-(((1R,2S)-1-hydroxy-1-(4-methoxybenzo[d][1,3]dioxol-6-yl)propan-2-yloxy)carbonyl)-3-hydroxybutan-2-yl octanoateC24H36O9[α]D = −28.16 (c 1.5, CHCl3),Source of chirality: asymmetric synthesisAbsolute configuration: (2S,3R,1′R,2′S)
(2S,3R)-3-(((1R,2S)-1-Hydroxy-1-(4-methoxybenzo[d][1,3]dioxol-6-yl)propan-2-yloxy)carbonyl)-3-hydroxybutan-2-yl hexanoateC22H32O9[α]D = −28.2 (c 1.5, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,3R,1′R,2′S)
(2S,3R)-3-(((1R,2S)-1-Hydroxy-1-(4-methoxybenzo[d][1,3]dioxol-6-yl)propan-2-yloxy)carbonyl)-3-hydroxybutan-2-yl butanoateC20H28O9[α]D = −29.1 (c 1.0, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2S,3R,1′R,2′S)
(R)-(4-Methoxybenzo[d][1,3]dioxol-6-yl)((S)-oxiran-2-yl) methanolC11H12O5[α]D = –25.6 (c 3.75, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (R)
(1R,2S)-3-(Diethylamino)-1-(4-methoxybenzo[a][1,3]dioxol-6-yl)propane-1,2-diolC15H23NO5[α]D = −9.0 (c 0.66, acetone)Source of chirality: asymmetric synthesisAbsolute configuration: (1R,2S)
(1R,2S)-1-(4-Methoxybenzo[a][1,3]dioxol-6-yl)-3-(propylamino)propane-1,2-diolC14H21NO5[α]D = −37.7 (c 0.2, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1R,2S)
(1R,2S)-3-(Isobutylamino)-1-(4-methoxybenzo[a][1,3]dioxol-6-yl)propane-1,2-diolC15H23NO5[α]D = −17.25 (c 0.16, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1R,2S)
(1R,2S)-3-(4-Methoxyphenylamino)-1-(4-methoxybenzo[a][1,3]dioxol-6-yl)propane-1,2-diolC19H23NO6[α]D = −22.9 (c 0.56, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (1R,2S)
(1R,2S)-3-(3-(Trifluoromethyl)benzylamino)-1-(4-methoxybenzo[a][1,3]dioxol-6-yl)propane-1,2-diolC19H20F3NO5[α]D = −21.1 (c 1.0, CHCl3)Source of chirality: Asymmetric synthesisAbsolute configuration: (1R,2S)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 4, 11 March 2009, Pages 440–448