| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347261 | 980302 | 2009 | 4 صفحه PDF | دانلود رایگان |
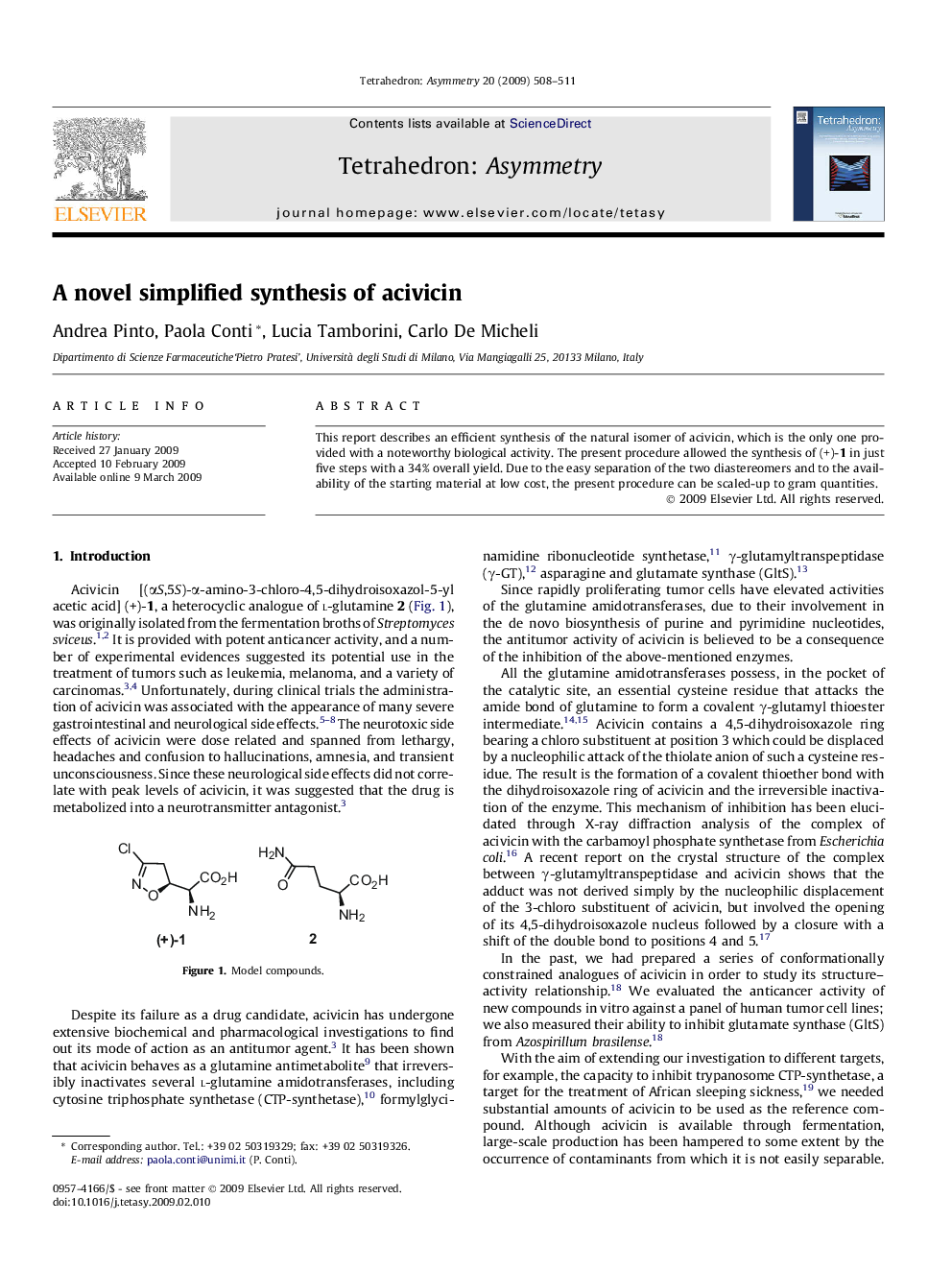
This report describes an efficient synthesis of the natural isomer of acivicin, which is the only one provided with a noteworthy biological activity. The present procedure allowed the synthesis of (+)-1 in just five steps with a 34% overall yield. Due to the easy separation of the two diastereomers and to the availability of the starting material at low cost, the present procedure can be scaled-up to gram quantities.
Figure optionsDownload as PowerPoint slide
(+)-(2R,5S)-2-Amino-2-(3-chloro-4,5-dihydro-isoxazol-5-yl)-ethanolC5H9ClN2O2[α]D20=+95.7 (c 1.00, CHCl3)Source of chirality: (R)-Garner’s aldehydeAbsolute configuration: (2R,5S)
(+)-(1R,5S)-[1-(3-Chloro-4,5-dihydro-isoxazol-5-yl)-2-hydroxy-ethyl]-carbamic acid tert-butyl esterC10H17ClN2O4[α]D20=+98.2 (c 1.00, CHCl3)Source of chirality: (R)-Garner’s aldehydeAbsolute configuration: (1R,5S)
(+)-[(αS,5S)-α-Amino-3-chloro-4,5-dihydroisoxazol-5-yl acetic acid]C5H7ClN2O3[α]D20=+157.6 (c 0.13, H2O)Source of chirality: (R)-Garner’s aldehydeAbsolute configuration: (αS,5S)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 4, 11 March 2009, Pages 508–511