| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347281 | 980303 | 2009 | 7 صفحه PDF | دانلود رایگان |
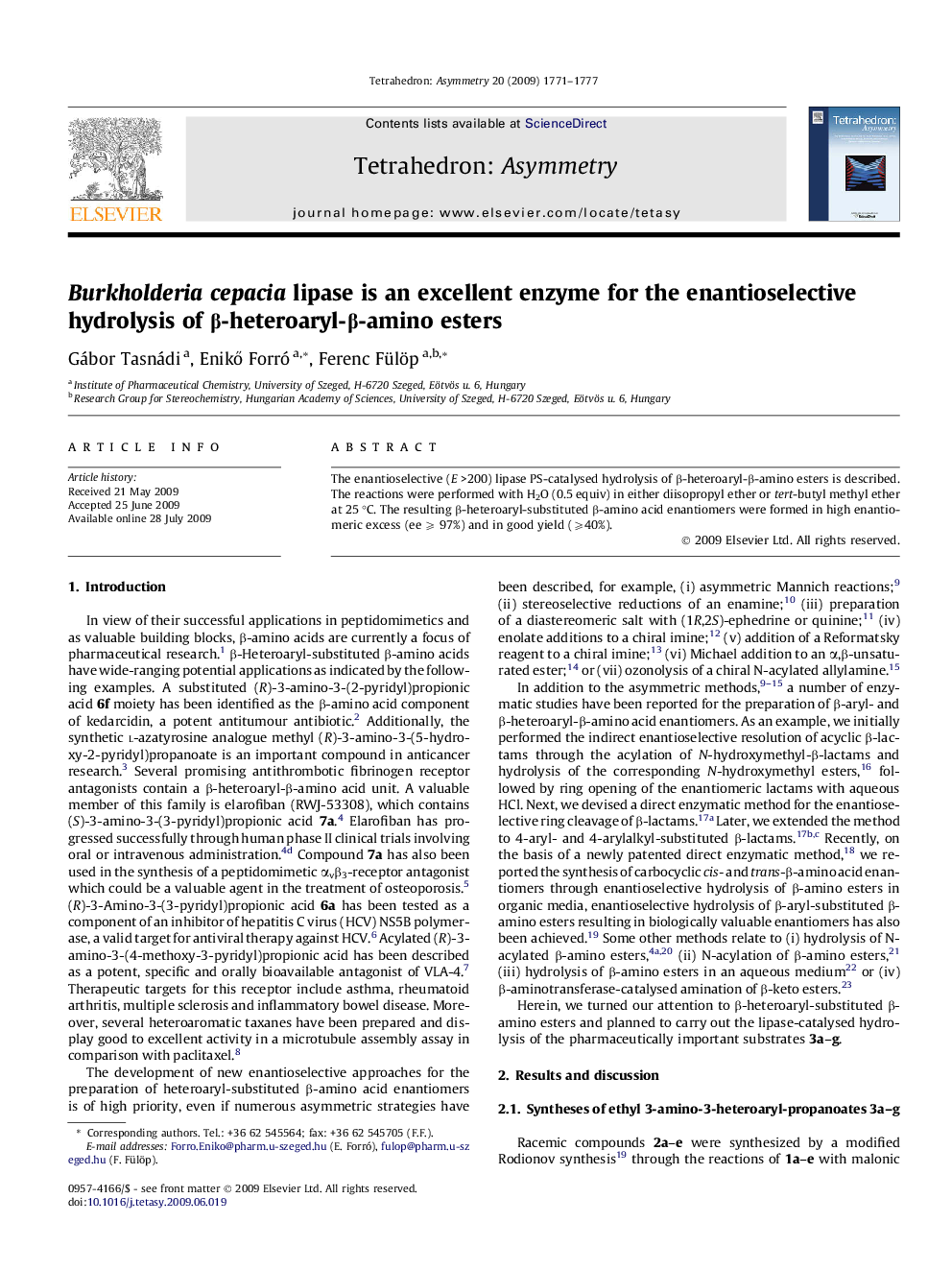
The enantioselective (E >200) lipase PS-catalysed hydrolysis of β-heteroaryl-β-amino esters is described. The reactions were performed with H2O (0.5 equiv) in either diisopropyl ether or tert-butyl methyl ether at 25 °C. The resulting β-heteroaryl-substituted β-amino acid enantiomers were formed in high enantiomeric excess (ee ⩾ 97%) and in good yield (⩾40%).
Figure optionsDownload as PowerPoint slide
(S)-3-Amino-3-(3-pyridyl)propanoic acidC8H10N2O2Ee >99% by HPLC on a Chirobiotic TAG column[α]D25=-5.1 (c 0.41, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(3-pyridyl)propanoic acid hydrochlorideC8H11ClN2O2Ee >99% by HPLC on a Chirobiotic TAG column[α]D25=-3.9 (c 0.33, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(2-furyl)propanoic acidC7H9NO3Ee >99% by HPLC on a Chirobiotic TAG column[α]D25=-5.8 (c 0.52, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(2-furyl)propanoic acid hydrochlorideC7H10ClNO3Ee >99% by HPLC on a Chirobiotic TAG column[α]D25=-4.9 (c 0.32, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(3-furyl)propanoic acidC7H9NO3Ee >99% by GC on a Chirasil L-Val column after derivatization with CH2N2 and (PrCO)2O[α]D25=-6.7 (c 0.34, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(3-furyl)propanoic acid hydrochlorideC7H10ClNO3Ee >99% by GC on a Chirasil L-Val column after derivatization with CH2N2 and (PrCO)2O[α]D25=-4.6 (c 0.42, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(2-thienyl)propanoic acidC7H9NO2SEe >99% by HPLC on a Chirobiotic TAG column[α]D25=-9.9 (c 0.41, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(2-thienyl)propanoic acid hydrochlorideC7H10ClNO2SEe >99% by HPLC on a Chirobiotic TAG column[α]D25=-3.1 (c 0.33, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(3-thienyl)propanoic acidC7H9NO2SEe >99% by GC on a Chirasil L-Val column after derivatization with CH2N2 and (EtCO)2O[α]D25=-3.2 (c 0.32, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(3-thienyl)propanoic acid hydrochlorideC7H10ClNO2SEe >99% by GC on a Chirasil L-Val column after derivatization with CH2N2 and (EtCO)2O[α]D25=-3.6 (c 0.34, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(2-pyridyl)propanoic acidC8H10N2O2Ee >99% by HPLC on a Chiralpak IA column after derivatization with CH2N2[α]D25=-18.2 (c 0.32, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(2-pyridyl)propanoic acid hydrochlorideC8H11ClN2O2Ee >99% by HPLC on a Chiralpak IA column after derivatization with CH2N2[α]D25=-8.6 (c 0.31, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(4-pyridyl)propanoic acidC8H10N2O2Ee = 98% by HPLC on a Chirobiotic TAG column[α]D25=-11.7 (c 0.36, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
(S)-3-Amino-3-(4-pyridyl)propanoic acid hydrochlorideC8H11ClN2O2Ee = 98% by HPLC on a Chirobiotic TAG column[α]D25=-3.6 (c 0.35, H2O)Source of chirality: lipase PS-catalyzed hydrolysisAbsolute configuration: (3S)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 15, 12 August 2009, Pages 1771–1777