| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347283 | 980303 | 2009 | 7 صفحه PDF | دانلود رایگان |
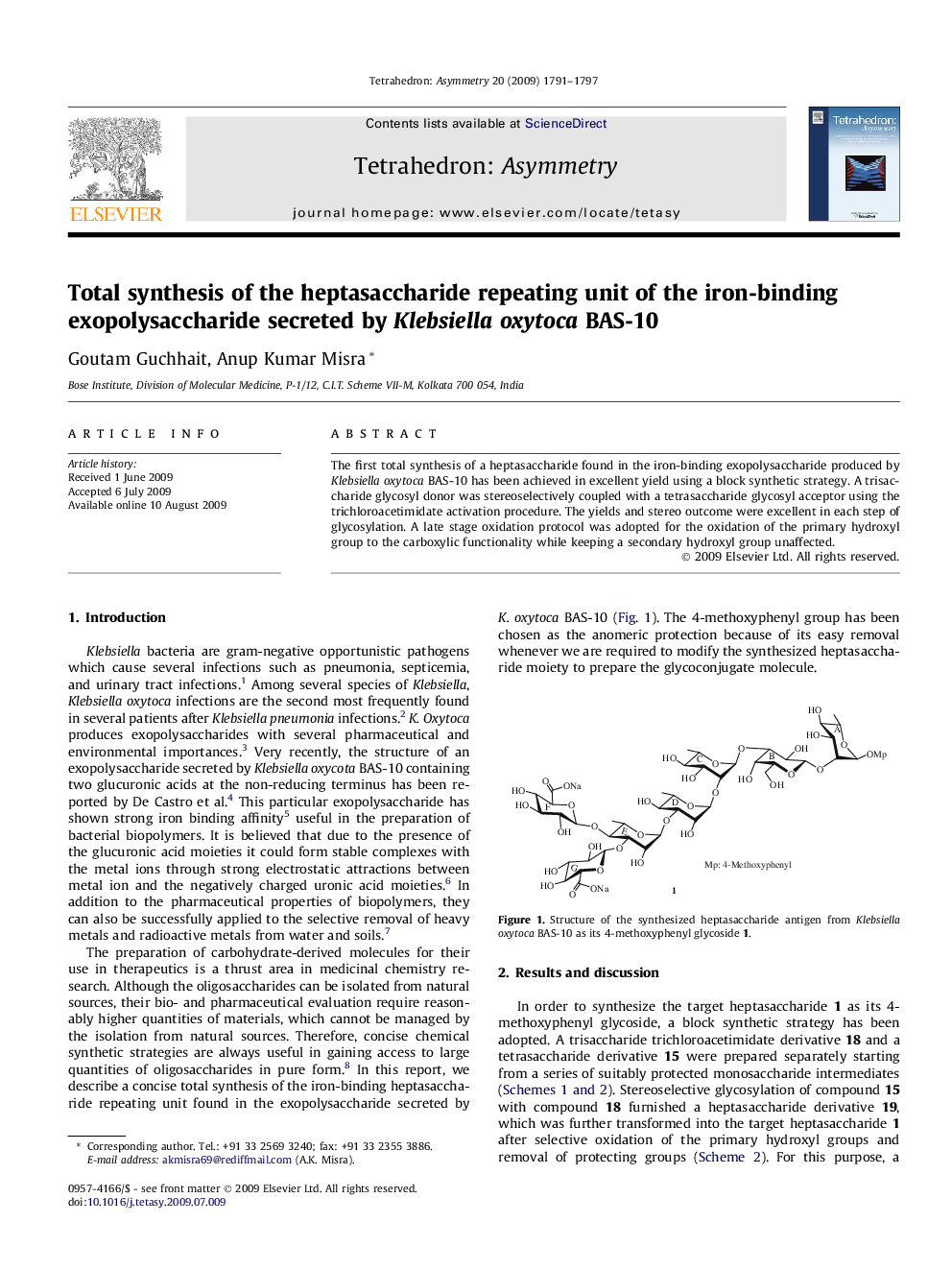
The first total synthesis of a heptasaccharide found in the iron-binding exopolysaccharide produced by Klebsiella oxytoca BAS-10 has been achieved in excellent yield using a block synthetic strategy. A trisaccharide glycosyl donor was stereoselectively coupled with a tetrasaccharide glycosyl acceptor using the trichloroacetimidate activation procedure. The yields and stereo outcome were excellent in each step of glycosylation. A late stage oxidation protocol was adopted for the oxidation of the primary hydroxyl group to the carboxylic functionality while keeping a secondary hydroxyl group unaffected.
Figure optionsDownload as PowerPoint slide
4-Methoxyphenyl 3,4-di-O-benzyl-α-l-rhamnopyranosideC27H30O6[α]D25=-43 (c 1.5, CHCl3)Source of chirality: l-rhamnose
4-Methoxyphenyl (2,4,6-tri-O-acetyl-3-O-allyl-β-d-galactopyranosyl)-(1→2)-3,4-di-O-benzyl-α-l-rhamnopyranosideC42H50O14[α]D25=+12.3 (c 1.5, CHCl3)Source of chirality: d-galactose, l-rhamnose
4-Methoxyphenyl (2-O-acetyl-3,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4,6-tri-O-benzyl-β-d-galactopyranosyl)-(1→2)-3,4-di-O-benzyl-α-l-rhamnopyranosideC76H82O16[α]D25=+2.6 (c 1.5, CHCl3)Source of chirality: d-galactose, l-rhamnose
4-Methoxyphenyl (2,3-di-O-acetyl-4-O-benzyl-α-l-rhamnopyranosyl)-(1→2)-(3,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4,6-tri-O-benzyl-β-d-galactopyranosyl)-(1→2)-3,4-di-O-benzyl-α-l-rhamnopyranosideC91H100O21[α]D25=-7.5 (c 1.0, CHCl3)Source of chirality: d-galactose, l-rhamnose
4-Methoxyphenyl (2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)-(1→3)-[(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)-(1→4)]-2-O-acetyl-α-l-rhamnopyranosideC43H56O25[α]D25=-2.3 (c 1.0, CHCl3)Source of chirality: d-glucose, l-rhamnose
4-Methoxyphenyl (2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)-(1→3)-[(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)-(1→4)]-2-O-acetyl-α-l-rhamnopyranosyl-(1→3)-(2-O-acetyl-4-O-benzyl-α-l-rhamnopyranosyl)-(1→2)-(3,4-di-O-benzyl-α-l-rhamnopyranosyl)-(1→3)-(2,4,6-tri-O-benzyl-β-d-galactopyranosyl)-(1→2)-3,4-di-O-benzyl-α-l-rhamnopyranosideC125H146O43[α]D25=+3.2 (c 1.5, CHCl3)Source of chirality: d-galactose, d-glucose, l-rhamnose
4-Methoxyphenyl (sodium β-d-glucopyranosyl uronate)-(1→3)-[(sodium β-d-glucopyranosyl uronate)-(1→4)]-α-l-rhamnopyranosyl-(1→3)-(α-l-rhamnopyranosyl)-(1→2)-(α-l-rhamnopyranosyl)-(1→3)-(β-d-galactopyranosyl)-(1→2)-α-l-rhamnopyranosideC49H72Na2O35[α]D25=-64 (c 1.0, CH3OH)Source of chirality: d-galactose, d-glucosamine, l-fucose
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 15, 12 August 2009, Pages 1791–1797