| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347287 | 980303 | 2009 | 4 صفحه PDF | دانلود رایگان |
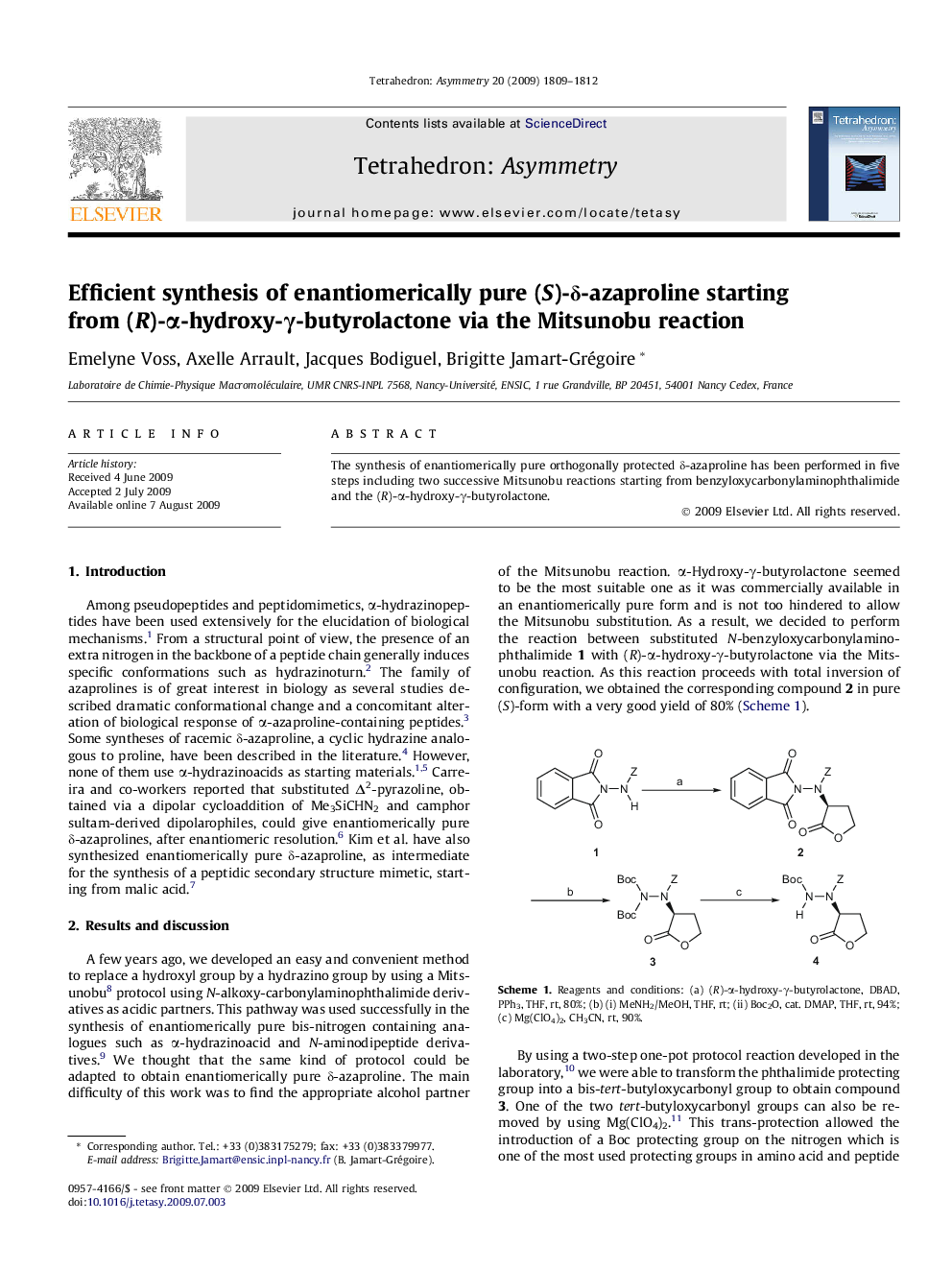
The synthesis of enantiomerically pure orthogonally protected δ-azaproline has been performed in five steps including two successive Mitsunobu reactions starting from benzyloxycarbonylaminophthalimide and the (R)-α-hydroxy-γ-butyrolactone.
Figure optionsDownload as PowerPoint slide
(S)-α-(N-Benzyloxycarbonylaminophthamilido)-γ-butyrolactoneC20H16N2O6[α]D22=-2.63 (c 0.760, EtOH)Source of chirality: (R)-α-hydroxy-γ-butyrolactoneAbsolute configuration: (S)
(S)-α-[Nα-(Benzyloxycarbonyl)-Nβ,Nβ-bis(tert-butyl-oxycarbonyl)hydrazino]-γ-butyrolactoneC22H30N2O8[α]D22=-2.54 (c 1.180, EtOH)Source of chirality: (R)-α-hydroxy-γ-butyrolactoneAbsolute configuration: (S)
(S)-α-[Nα-(Benzyloxycarbonyl)-Nβ-(tert-butyloxy-carbonyl)-hydrazino]-γ-butyrolactoneC17H22N2O6[α]D22=-2.86 (c 1.050, EtOH)Source of chirality: (R)-α-hydroxy-γ-butyrolactoneAbsolute configuration: (S)
Isopropyl (2S)-2-[Nα-(benzyloxycarbonyl)-Nβ-(tert-butyloxycarbonyl)hydrazino]-4-hydroxybutanoateC20H30N2O7[α]D22=-0.74 (c 1.360, EtOH)Source of chirality: (R)-α-hydroxy-γ-butyrolactoneAbsolute configuration: (2S)
Isopropyl (3S)-3-[2-(benzyloxycarbonyl)-(tert-butyl-oxycarbonyl)pyrazolidine]-carboxylateC20H28N2O6[α]D22=-12.94 (c 0.850, EtOH)Source of chirality: (R)-α-hydroxy-γ-butyrolactoneAbsolute configuration: (3S)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 15, 12 August 2009, Pages 1809–1812