| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347379 | 980307 | 2008 | 9 صفحه PDF | دانلود رایگان |
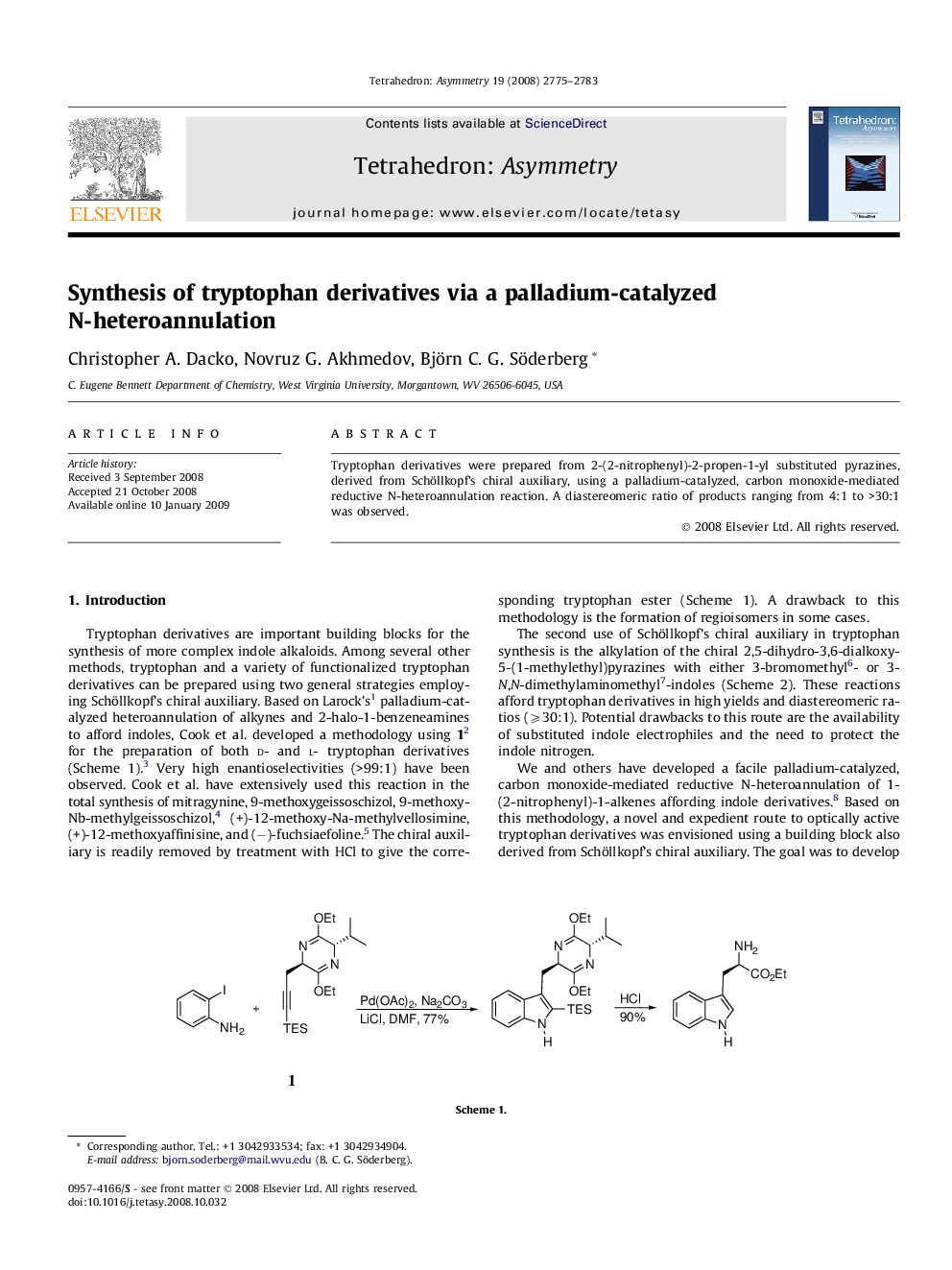
Tryptophan derivatives were prepared from 2-(2-nitrophenyl)-2-propen-1-yl substituted pyrazines, derived from Schöllkopf’s chiral auxiliary, using a palladium-catalyzed, carbon monoxide-mediated reductive N-heteroannulation reaction. A diastereomeric ratio of products ranging from 4:1 to >30:1 was observed.
Figure optionsDownload as PowerPoint slide
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-2-(2-trimethylstannyl-2-propen-1-yl)pyrazineC15H28N2O2SnDe ⩾ 93.5%[α]D25=-12.0 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
Bis-2,3-((2R,5S)-2,5-dihydro-3,6-dimethoxy-5-methylethyl-2-pyrazinylmethyl)-1,4-butadieneC24H38N4O4De ⩾ 93.5%[α]D25=-50.8 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-2-(2-(4-methoxy-2-nitrophenyl)-2-propen-1-yl)-5-(1-methylethyl)-3,6-dimethoxy-pyrazineC19H25N3O5De ⩾ 93.5%[α]D25=-20.2 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-(2-(5-methoxy-2-nitrophenyl)-2-propen-1-yl)-5-(1-methylethyl)pyrazineC19H25N3O5De ⩾ 93.5%[α]D25=-17.5 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-(2-(4-fluoro-2-nitrophenyl)-2-propen-1-yl)-5-(1-methylethyl)pyrazineC18H22FN3O4De ⩾ 93.5%[α]D25=-26.5 (c 1.4, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
Methyl 4-(1-((2R,5S)-2,5-dihydro-5-(1-methylethyl)-3,6-dimethoxypyrazin-2-yl)prop-2-en-1-yl)-3-nitrobenzoateC20H25N3O6De ⩾ 93.5%[α]D25=-8.1 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-(2-(2,4-dinitrophenyl)-2-propen-1-yl)-5-(1-methylethyl)pyrazineC18H22N4O6De ⩾ 93.5%[α]D25=-4.3 (c 1.1, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-(2-(3-nitropyridin-2-yl)prop-2-en-1-yl)-5-(1-methylethyl)pyrazineC17H22N4O4De ⩾ 93.5%[α]D25=+4.8 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-(2-(2-methoxy-6-nitrophenyl)prop-2-en-1-yl)-5-(1-methylethyl)pyrazineC19H25N3O5De ⩾ 93.5%[α]D25=-27.2 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-(2-(3-methoxy-2-nitrophenyl)prop-2-en-1-yl)-5-(1-methylethyl)pyrazineC19H25N3O5De ⩾ 93.5%[α]D25=+3.72 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2-(3-Bromo-3-buten-1-yl)-2,5-dihydro-3,6-dimethoxy-5-methylethylpyrazineC13H21BrN2O2De ⩾ 93.5%[α]D25=-2.3 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2-(3-Bromo-2-propen-1-yl)-2,5-dihydro-3,6-dimethoxy-5-methylethylpyrazineC12H19BrN2O2De ⩾ 93.5% as a cis/trans mixture[α]D25=-14.4 (c 1.4, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-(3-(2-nitrophenyl)-3-buten-1-yl)-5-(1-methylethyl)pyrazineC19H25N3O4De ⩾ 93.5%[α]D25=+4.8 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-2-((2-nitrophenyl)-2-propen-1-yl))-5-(1-methylethyl)pyrazineC18H23N3O4De ⩾ 93.5% as a cis/trans mixture[α]D25=-7.7 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-6-methoxy-1H-indoleC19H25N3O3De = 85%, rest (2S,5S)-diastereomer[α]D25=-44.6 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-5-methoxy-1H-indoleC19H25N3O3De = 91%, rest (2S,5S)-diastereomer[α]D25=-49.5 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
6-Fluoro-3-(((2R,5S)-2,5-dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-1H-indoleC18H22FN3O2De = 64%, rest (2S,5S)-diastereomer[α]D25=-41.8 (c 0.9, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
Methyl 3-(((2R,5S)-2,5-dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-1H-indole-6-carboxylateC20H25N3O4De = 82%, rest (2S,5S)-epimer[α]D25=-43.1 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-6-nitro-1H-indoleC18H22N4O4De ⩾ 93.5%[α]D25=-36.6 (c 1.3, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-1H-pyrrolo[3,2-b]pyridineC17H22N4O2De = 83%, rest (2S,5S)-diastereomer[α]D25=-23.2 (c 1.1, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-4-methoxy-1H-indoleC19H25N3O3De ⩾ 93.5%[α]D25=-22.3 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-7-methoxy-1H-indoleC19H25N3O3De = 71%, rest (2S,5S)-diastereomer[α]D25=-25.2 (c 1.1, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(2-((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)ethyl)-1H-indoleC19H25N3O2De = 88%, rest (2S,5S)-diastereomer[α]D25=-2.7 (c 0.8, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
2-((2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-1H-indoleC18H23N3O2De = 9%, rest (2S,5S)-epimer[α]D25=+0.35 (c 1.0, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
2-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-pyrazin-2-yl)methyl)-1-hydroxyindoleC18H23N3O3De ⩾ 93.5%[α]D25=-8.5 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
(2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)-2-(2-(2-nitrophenyl)-2-propen-1-yl)pyrazineC18H23N3O4De = 88%, rest (2S,5S)-diastereomer[α]D25=-21.8 (c 1.2, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
3-(((2R,5S)-2,5-Dihydro-3,6-dimethoxy-5-(1-methylethyl)pyrazin-2-yl)methyl)-1H-indoleC18H23N3O2De = 88%, rest (2S,5S)-epimer[α]D25=-54.8 (c 1.3, CH2Cl2)Source of chirality: l-valineAbsolute configuration: (2R,5S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 24, 12 December 2008, Pages 2775–2783