| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347381 | 980307 | 2008 | 7 صفحه PDF | دانلود رایگان |
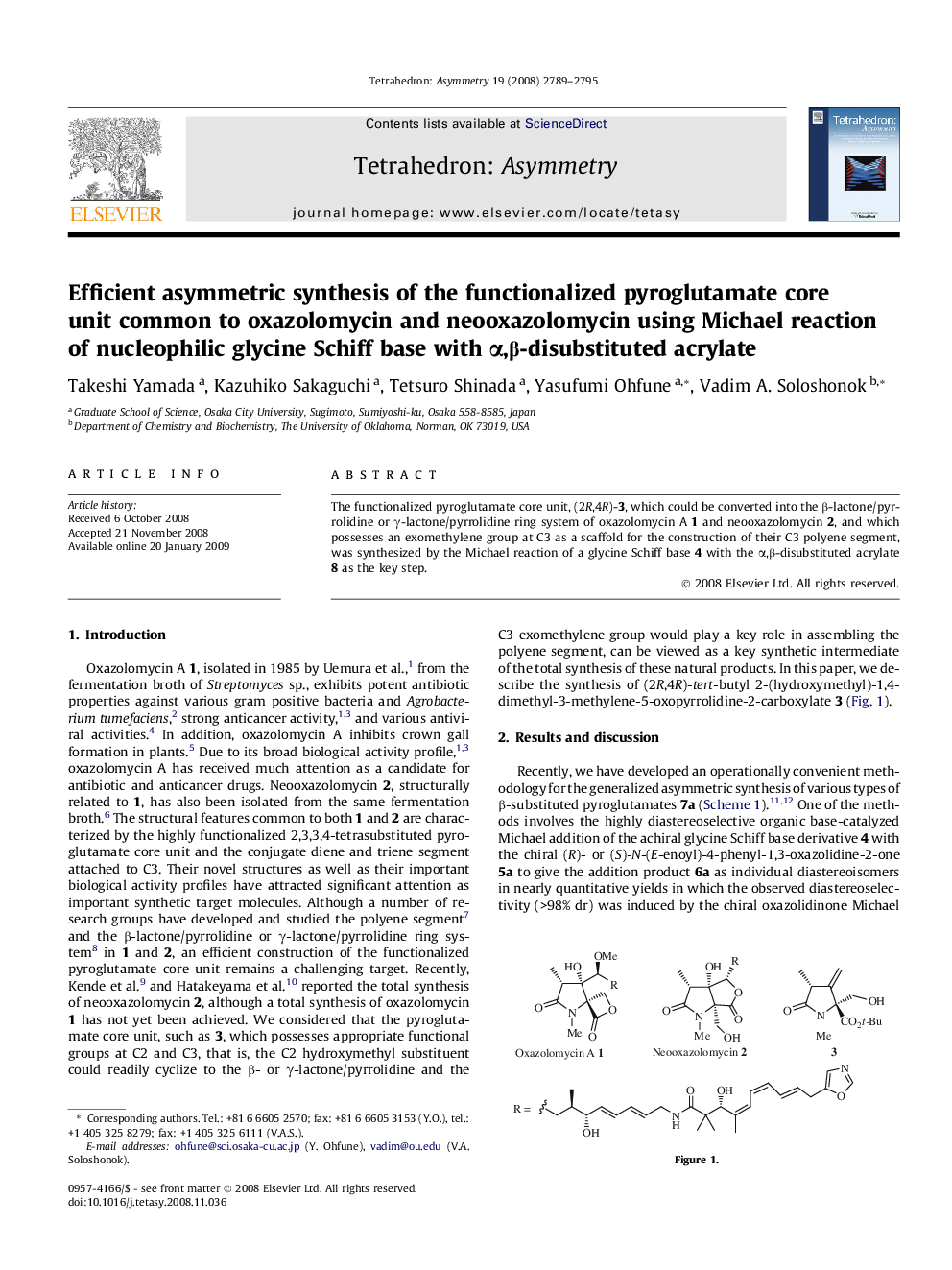
The functionalized pyroglutamate core unit, (2R,4R)-3, which could be converted into the β-lactone/pyrrolidine or γ-lactone/pyrrolidine ring system of oxazolomycin A 1 and neooxazolomycin 2, and which possesses an exomethylene group at C3 as a scaffold for the construction of their C3 polyene segment, was synthesized by the Michael reaction of a glycine Schiff base 4 with the α,β-disubstituted acrylate 8 as the key step.
Figure optionsDownload as PowerPoint slide
(R)-3-((E)-4-(Benzyloxy)but-2-enoyl)-4-phenyloxazolidin-2-oneC20H19NO4[α]D20.9=-69.9 (c 1.07, CHCl3)Source of chirality: (R)-α-phenylglycineAbsolute configuration: (R)
(2R,3R)-Methyl 3-((benzyloxy)methyl)-5-oxopyrrolidine-2-carboxylateC14H17NO4[α]D27.0=-27.3 (c 1.0, CHCl3)Source of chirality: (R)-α-phenylglycineAbsolute configuration: (2R,3R)
(S)-3-((E)-4-(Benzyloxy)-2-methylbut-2-enoyl)-4-phenyloxazolidin-2-oneC21H21NO4[α]D20.5=+12.9 (c 0.77, CHCl3)Source of chirality: (R)-α-phenylglycineAbsolute configuration: (S)
N-(2-Benzoyl-phenyl)-2-piperidyl-acetamide Ni(II) complex of (2S,3S,4R,4′S)-3-benzyloxymethyl-4-methyl-5-[3′-(4′-phenyl-2′-oxazolidinonyl)] glutamic acid schiff baseC43H44N4O7Ni[α]D21.2=+2376.3 (c 0.4, CHCl3)Source of chirality: (S)-4-phenyloxazolidin-2-oneAbsolute configuration: (2S,3S,4R,4′S)
(2S,3S,4R)-3-((Benzyloxy)methyl)-4-methyl-5-oxopyrrolidine-2-carboxylic acidC14H17NO4[α]D25.7=+24.3 (c 1.0, CHCl3)Source of chirality: (–)-verbenone and stereoselective synthesisAbsolute configuration: (2S,3S,4R)
(2S,3S,4R)-tert-Butyl 3-((benzyloxy)methyl)-4-methyl-5-oxopyrrolidine-2-carboxylateC18H25NO4[α]D25.4=+14.9 (c 1.4, CHCl3)Source of chirality: (–)-verbenone and stereoselective synthesisAbsolute configuration: (2S,3S,4R)
(2S,3S,4R)-tert-Butyl-3-((benzyloxy)methyl)-1,4-dimethyl-5-oxopyrrolidine-2-carboxylateC19H27NO4[α]D26.1=+25.8 (c 1.1, CHCl3)Source of chirality: (–)-verbenone and stereoselective synthesisAbsolute configuration: (2S,3S,4R)
((2S,3S,4R)-2-(tert-Butoxycarbonyl)-1,4-dimethyl-5-oxopyrrolidin-3-yl)methyl methyl carbonateC14H23NO6[α]D27.0=+27.5 (c 3.4, CHCl3)Absolute configuration: (2S,3S,4R)
(3R,3aS,6aR)-tert-Butyl-hexahydro-1,3-dimethyl-2,6-dioxo-1H-furo[3,4-b]pyrrole-6a-carboxylateC13H19NO5[α]D25.1=+88.7 (c 1.8, CHCl3)Absolute configuration: (3R,3aS,6aR)
(2R,3S,4R)-2-tert-butyl 2-methyl 1,4-dimethyl-5-oxo-3-((phenylselanyl)methyl) pyrrolidine-2,2-dicarboxylateC20H27NO5Se[α]D26.7=-52.8 (c 0.9, CHCl3)Absolute configuration: (2R,3S,4R)
(2R,4R)-2-tert-Butyl 2-methyl 1,4-dimethyl-3-methylene-5-oxopyrrolidine-2,2-dicarboxylateC14H21NO5[α]D25=-84.0 (c 1.3, CHCl3)Absolute configuration: (2R,4R)
(2R,4R)-tert-Butyl 2-(hydroxymethyl)-1,4-dimethyl-3-methylene-5-oxopyrrolidine-2-carboxylateC13H21NO4[α]D25=-85.7 (c 1.2, CHCl3)Absolute configuration: (2R,3S,4R)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 24, 12 December 2008, Pages 2789–2795