| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347474 | 980310 | 2009 | 4 صفحه PDF | دانلود رایگان |
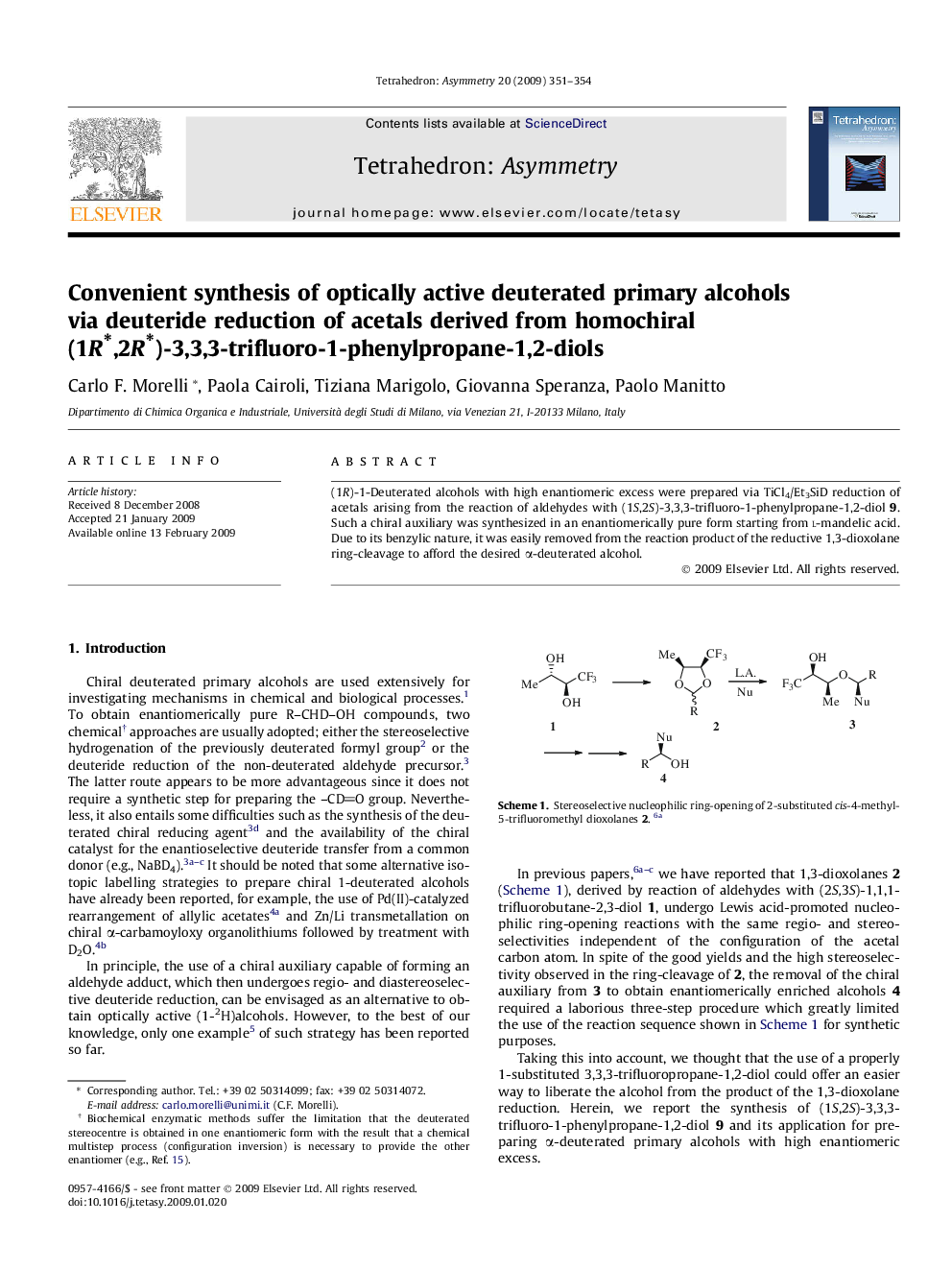
(1R)-1-Deuterated alcohols with high enantiomeric excess were prepared via TiCl4/Et3SiD reduction of acetals arising from the reaction of aldehydes with (1S,2S)-3,3,3-trifluoro-1-phenylpropane-1,2-diol 9. Such a chiral auxiliary was synthesized in an enantiomerically pure form starting from l-mandelic acid. Due to its benzylic nature, it was easily removed from the reaction product of the reductive 1,3-dioxolane ring-cleavage to afford the desired α-deuterated alcohol.
Figure optionsDownload as PowerPoint slide
(1S,2S)-3,3,3-Trifluoro-1-phenylpropane-1,2-diolC9H9F3O2Ee > 99%[α]D=+14.0[α]D=+14.0 (c 1.06, MeOH)Source of chirality: chiral starting materialAbsolute configuration: (1S,2S)
(2S,4S,5S)-2-Heptyl-4-phenyl-5-(trifluoromethyl)-1,3-dioxolaneC17H23F3O2De > 99%[α]D=+101.0[α]D=+101.0 (c 1.09, EtOH)Source of chirality: chiral starting materialAbsolute configuration: (2S,4S,5S)
(2S,4S,5S)-2-Phenethyl-4-phenyl-5-(trifluoromethyl)-1,3-dioxolaneC18H17F3O2De > 99%[α]D=+120.7[α]D=+120.7 (c 0.93, EtOH)Source of chirality: chiral starting materialAbsolute configuration: (2S,4S,5S)
(2S,4S,5S)-2-Benzyl-4-phenyl-5-(trifluoromethyl)-1,3-dioxolaneC17H15F3O2De > 99%[α]D=+121.3[α]D=+121.3 (c 0.95, EtOH)Source of chirality: chiral starting materialAbsolute configuration: (2S,4S,5S)
(2S,4S,5S)-2-Cyclohexyl-4-phenyl-5-(trifluoromethyl)-1,3-dioxolaneC16H19F3O2De > 99%[α]D=+72.8[α]D=+72.8 (c 0.99, EtOH)Source of chirality: chiral starting materialAbsolute configuration: (2S,4S,5S)
(2S,3S)-1,1,1-Trifluoro-3-((R)-[α-2H]octyloxy)-3-phenylpropan-2-olC17H24DF3O2[α]D=+17.5[α]D=+17.5 (c 1.10, MeOH)Diastereomeric ratio at deuterated carbon = 95:5Source of chirality at deuterated carbon: asymmetric inductionAbsolute configuration at deuterated carbon: (R)
(2S,3S)-1,1,1-Trifluoro-3-phenyl-3-((R)-[α-2H]3-phenylpropyloxy)propan-2-olC18H18DF3O2[α]D=+43.1[α]D=+43.1 (c 0.73, MeOH)Diastereomeric ratio at deuterated carbon = 94:6Source of chirality at deuterated carbon: asymmetric inductionAbsolute configuration at deuterated carbon: (R)
(2S,3S)-1,1,1-Trifluoro-3-((R)-[α-2H]phenethoxy)-3-phenylpropan-2-olC17H16DF3O2[α]D=+55.6[α]D=+55.6 (c 0.62, MeOH)Diastereomeric ratio at deuterated carbon = 92:8Source of chirality at deuterated carbon: asymmetric inductionAbsolute configuration at deuterated carbon: (R)
(2S,3S)-3-((R)-[α-2H]Cyclohexylmethoxy)-1,1,1-trifluoro-3-phenylpropan-2-olC16H20DF3O2[α]D=+37.2[α]D=+37.2 (c 0.88, MeOH)Diastereomeric ratio at deuterated carbon = 96:4Source of chirality at deuterated carbon: asymmetric inductionAbsolute configuration at deuterated carbon: (R)
Journal: Tetrahedron: Asymmetry - Volume 20, Issue 3, 26 February 2009, Pages 351–354