| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347489 | 980311 | 2013 | 8 صفحه PDF | دانلود رایگان |
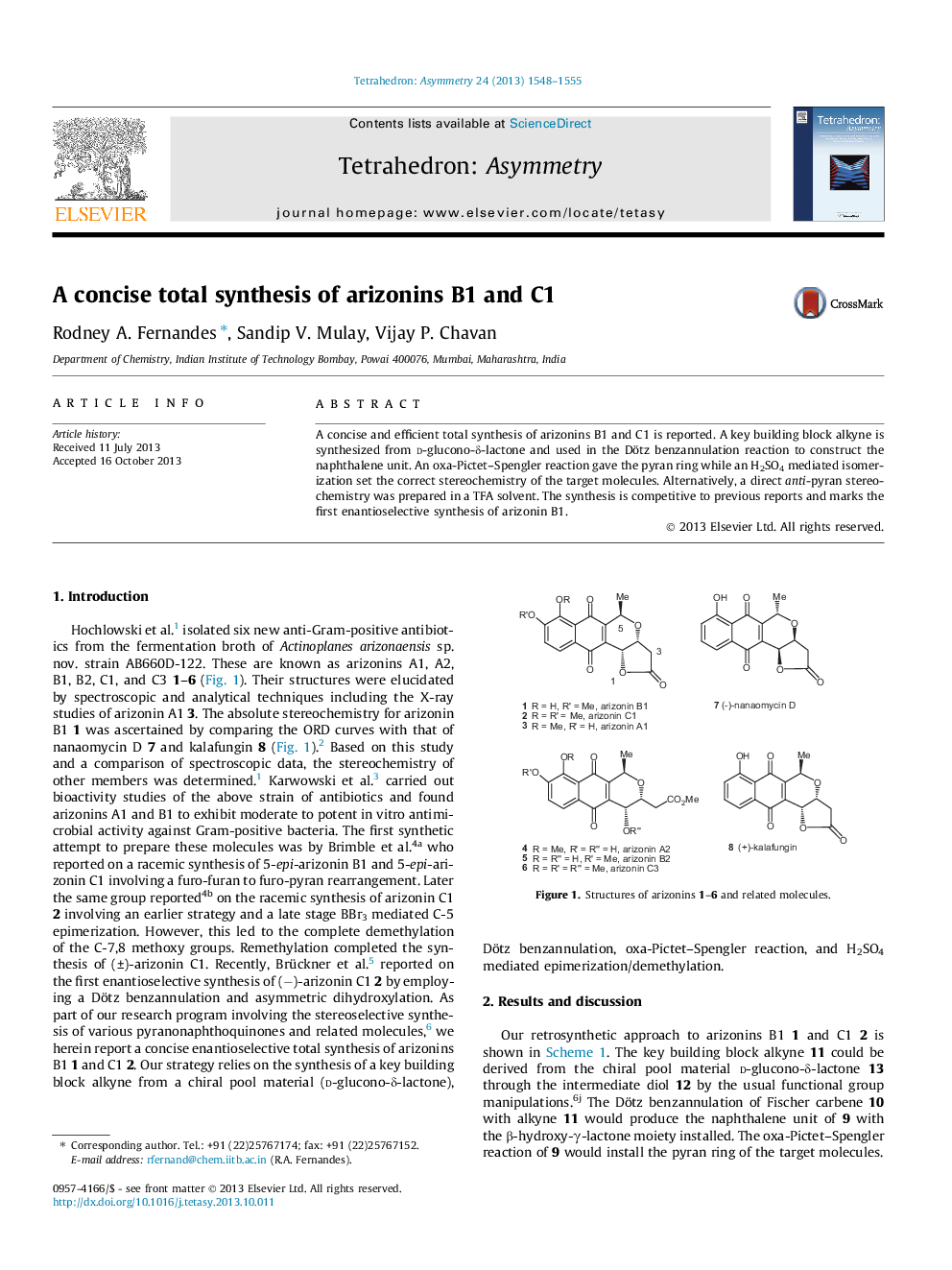
A concise and efficient total synthesis of arizonins B1 and C1 is reported. A key building block alkyne is synthesized from d-glucono-δ-lactone and used in the Dötz benzannulation reaction to construct the naphthalene unit. An oxa-Pictet–Spengler reaction gave the pyran ring while an H2SO4 mediated isomerization set the correct stereochemistry of the target molecules. Alternatively, a direct anti-pyran stereochemistry was prepared in a TFA solvent. The synthesis is competitive to previous reports and marks the first enantioselective synthesis of arizonin B1.
Figure optionsDownload as PowerPoint slide
Methyl (3R,4R)-3,4-isopropylidenedioxyhex-5-ynoateC10H14O4[α]D25=+1.65 (c 0.9, CHCl3)Initial source of chirality: d-glucono-δ-lactoneAbsolute configuration: (3R,4R)
Methyl-(3R,4R)-4-(1-hydroxy-4,5,6-trimethoxynaphthalen-2-yl)-3,4-isopropylidenedioxybutanoateC21H26O8[α]D25=+9.1 (c 0.6, CHCl3)Initial source of chirality: d-glucono-δ-lactoneAbsolute configuration: (3R,4R)
Methyl-(3R,4R)-4-(1,4,5,6-tetramethoxynaphthalen-2-yl)-3,4-isopropylidenedioxybutanoateC22H28O8[α]D25=+26.1 (c 0.6, CHCl3)Initial source of chirality: d-glucono-δ-lactoneAbsolute configuration: (3R,4R)
(4R,5R)-4-Hydroxy-5-(1,4,5,6-tetramethoxynaphth-2-yl)dihydrofuran-2(3H)-oneC18H20O7[α]D25=-4.6 (c 0.5, CHCl3)Initial source of chirality: d-glucono-δ-lactoneAbsolute configuration: (4R,5R)
(3aR,5R,11bR)-6,7,8,11-Tetramethoxy-5-methyl-3,3a,5,11b-tetrahydro-2H-benzo[g]furo[3,2-c]isochromen-2-oneC20H22O7[α]D25=+120.8 (c 0.25, CHCl3)Initial source of chirality: d-glucono-δ-lactoneAbsolute configuration: (3aR,5R,11bR)
Arizonin C1C18H16O7[α]D25=+75.3 (c 0.7, MeOH)Initial source of chirality: d-glucono-δ-lactoneAbsolute configuration: (3aR,5R,11bR)
Arizonin B1C17H14O7[α]D25=+137 (c 0.108, MeOH)Initial source of chirality: d-glucono-δ-lactoneAbsolute configuration: (3aR,5R,11bR)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 24, 31 December 2013, Pages 1548–1555