| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347493 | 980311 | 2013 | 4 صفحه PDF | دانلود رایگان |
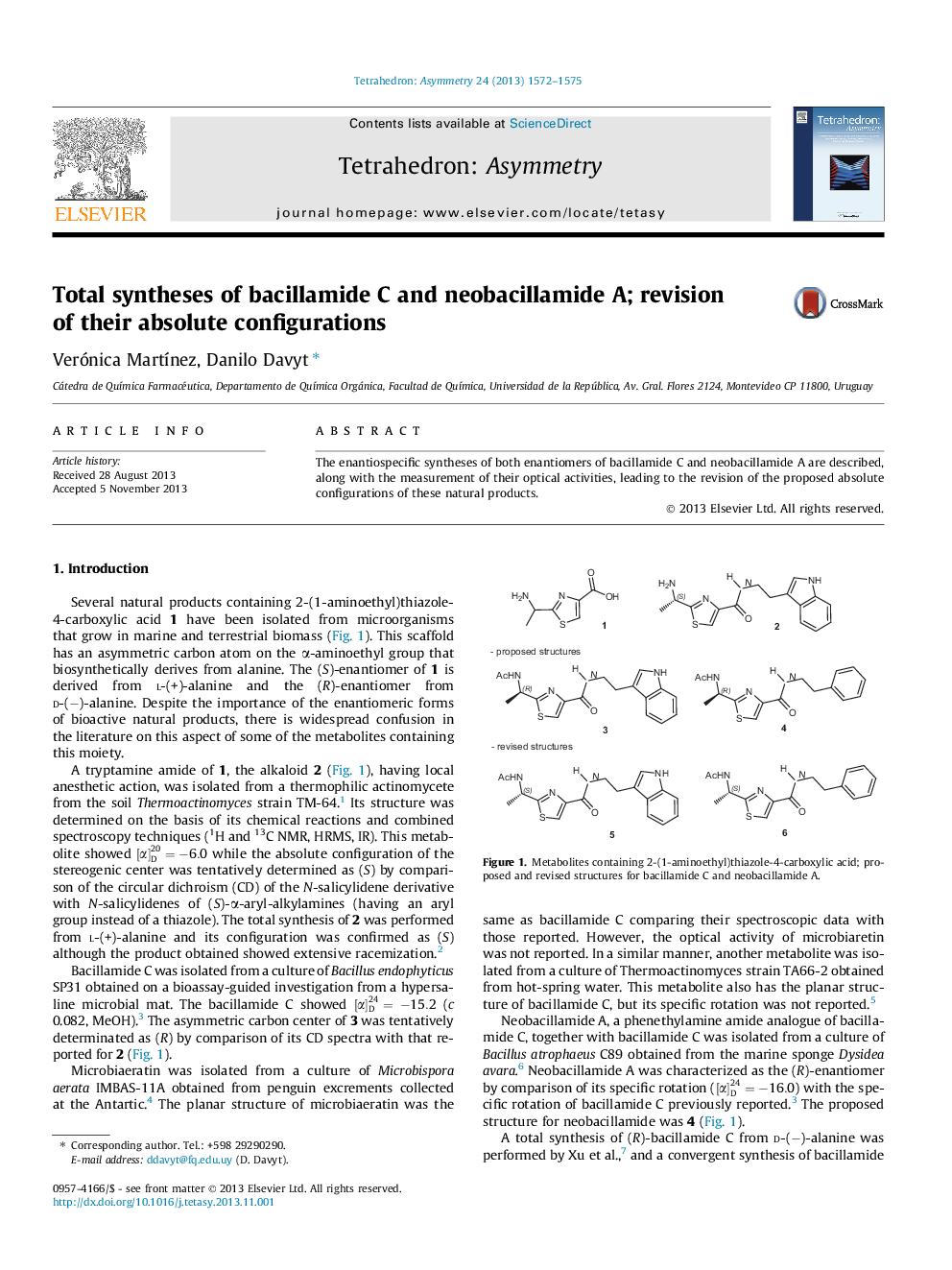
The enantiospecific syntheses of both enantiomers of bacillamide C and neobacillamide A are described, along with the measurement of their optical activities, leading to the revision of the proposed absolute configurations of these natural products.
Figure optionsDownload as PowerPoint slide
(R)-Ethyl 2-(1-(tert-butoxycarbonylamino)ethyl)thiazole-4-carboxylateC13H20N2O4See% = 93%[α]D24=+11.3 (c 0.65, CHCl3)Source of chirality: Stereospecific synthesisAbsolute configuration: (R)
(R)-Ethyl 2-(1-acetamidoethyl)thiazole-4-carboxylateC10H14N2O3S[α]D24=+29.1 (c 0.47, CHCl3)Source of chirality: Stereospecific synthesisAbsolute configuration: (R)
(R)-(N-(2-(1H-Indol-3-yl)ethyl)-2-(1-acetamidoethyl)thiazole-4-carboxamideC18H20N4O2See% >99%[α]D24=+23.1 (c 4.71, MeOH)Source of chirality: Stereospecific synthesisAbsolute configuration: (R)
(R)-(N-(2-Phenethyl)-2-(1-acetamidoethyl)thiazole-4-carboxamideC16H19N3O2S[α]D24=+22.5 (c 7.25, MeOH)Source of chirality: Stereospecific synthesisAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 24, 31 December 2013, Pages 1572–1575