| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347494 | 980311 | 2013 | 7 صفحه PDF | دانلود رایگان |
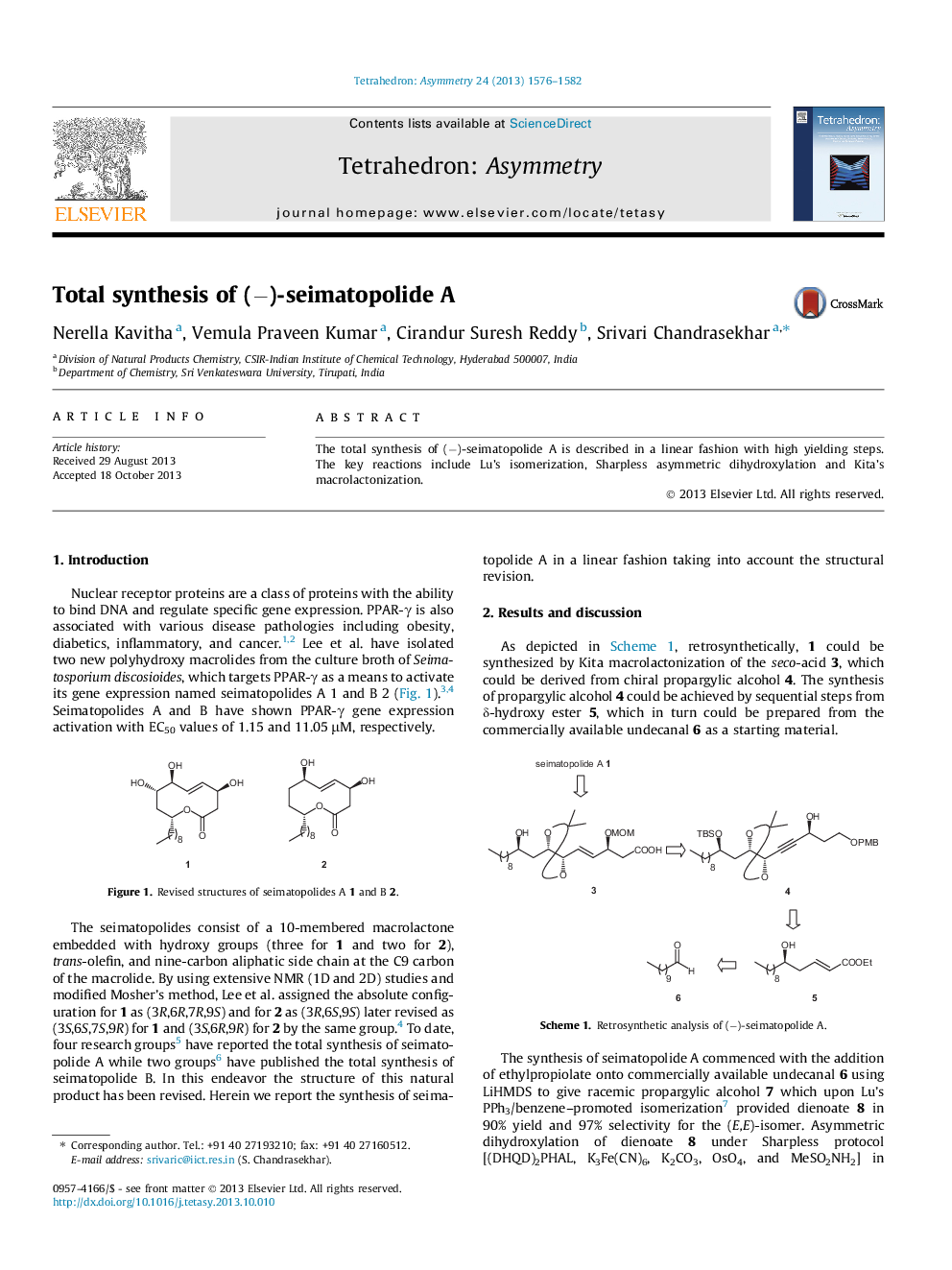
The total synthesis of (−)-seimatopolide A is described in a linear fashion with high yielding steps. The key reactions include Lu’s isomerization, Sharpless asymmetric dihydroxylation and Kita’s macrolactonization.
Figure optionsDownload as PowerPoint slide
(E)-Ethyl 3-((4R,5R)-5-nonyl-2-oxo-1,3-dioxolan-4-yl)acrylateC16H30O4[α]D25=+25.65 (c 1.34, CHCl3)Source of chirality: asymmetric dihydroxylationAbsolute configuration: (4R,5R)
(E)-Ethyl 3-((4R,5R)-5-nonyl-2-oxo-1,3-dioxolan-4-yl) acrylateC17H28O5[α]D25=+36.5 (c 3.5, CHCl3)Source of chirality: asymmetric dihydroxylationAbsolute configuration: (4R,5R)
Ethyl (R,E)-5-hydroxytetradec-2-enoateC16H30O3[α]D25=-2.05 (c 1.7, CHCl3)Source of chirality: asymmetric reductive hydrogenationAbsolute configuration: (5R)
Ethyl (R,E)-5-(tert-butyldimethylsilyloxy)tetradec-2-enoateC22H44O3Si[α]D25=+4.2 (c 2.72, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (5R)
Ethyl (2R,3S,5R)-5-(tert-butyldimethylsilyloxy)-2,3-dihydroxy tetradecanoateC22H46O5Si[α]D25=-112.8 (c 10.3, CHCl3)Source of chirality: asymmetric dihydroxylationAbsolute configuration: (2R,3S,5R)
Ethyl (4R,5S)-5-((R)-2-(tert-butyldimethylsilyloxy)undecyl)-2,2-dimethyl-1,3-dioxolane-4-carboxylateC25H50O5Si[α]D25=-24.2 (c 0.64, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3S,5R)
tert-Butyl((R)-1-((4S,5S)-5-ethynyl-2,2-dimethyl-1,3-dioxolan-4-yl)undecan-2-yloxy)dimethylsilaneC24H46O3Si[α]D25=-18.4 (c 4.77, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,4S,6R)
1-((4S,5S)-5-((R)-2-(tert-Butyldimethylsilyloxy)undecyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-5-(4-methoxybenzyloxy)pent-1-yn-3-oneC35H58O6Si[α]D25=-15.5 (c 1.06, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (6S,7S,9R)
(S)-1-((4S,5S)-5-((R)-2-(tert-Butyldimethylsilyloxy)undecyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-5-(4-methoxybenzyloxy)pent-1-yn-3-olC35H60O6Si[α]D25=-10.8 (c 1.06, CHCl3)Source of chirality: Noyori reductionAbsolute configuration: (3S,6S,7S,9R)
(S,E)-1-((4S,5S)-5-((R)-2-(tert-Butyldimethylsilyloxy)undecyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-5-(4-methoxybenzyloxy)pent-1-en-3-olC35H62O6Si[α]D25=-3.5 (c 0.85, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,6S,7S,9R)
tert-Butyl((R)-1-((4S,5S)-5-((S,E)-5-(4-methoxybenzyloxy)-3-(methoxymethoxy)pent-1-enyl)-2,2-dimethyl-1,3-dioxolan-4-yl)undecan-2-yloxy)dimethylsilaneC37H66O7Si[α]D25=-19.5 (c 1.04, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,6S,7S,9R)
(S,E)-5-((4S,5S)-5-((R)-2-(tert-Butyldimethylsilyloxy)undecyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-3-(methoxymethoxy)pent-4-en-1-olC29H58O6Si[α]D25==-50.9 (c 1.73, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,6S,7S,9R)
(S,E)-5-((4S,5S)-5-((R)-2-(tert-Butyldimethylsilyloxy)undecyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-3-(methoxymethoxy)pent-4-enoic acidC29H56O7Si[α]D25=-29.2 (c 1.04, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,6S,7S,9R)
(S,E)-5-((4S,5S)-5-((R)-2-Hydroxyundecyl)-2,2-dimethyl-1,3-dioxolan-4-yl)-3-(methoxymethoxy)pent-4-enoic acidC23H42O7[α]D25=-15.1 (c 1.22, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,6S,7S,9R)
(3aS,5R,9S,11aS,E)-9-(Methoxymethoxy)-2,2-dimethyl-5-nonyl-4,5,8,9-tetrahydro-3aH-[1,3]dioxolo[4,5-d]oxecin-7(11aH)-oneC23H40O6[α]D25=+21.85 (c 1.51, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (3S,6S,7S,9R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issue 24, 31 December 2013, Pages 1576–1582