| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347547 | 980313 | 2008 | 4 صفحه PDF | دانلود رایگان |
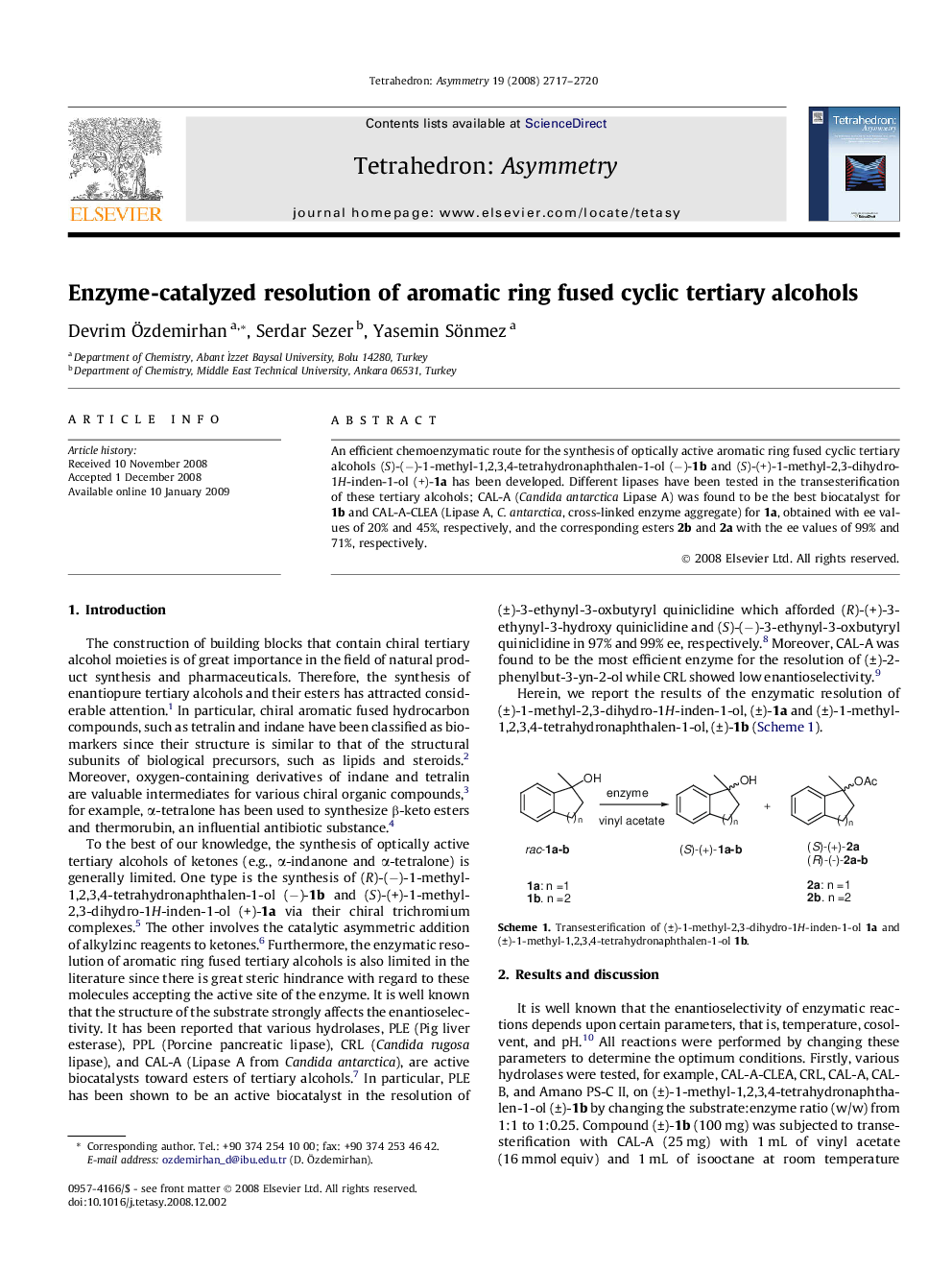
An efficient chemoenzymatic route for the synthesis of optically active aromatic ring fused cyclic tertiary alcohols (S)-(−)-1-methyl-1,2,3,4-tetrahydronaphthalen-1-ol (−)-1b and (S)-(+)-1-methyl-2,3-dihydro-1H-inden-1-ol (+)-1a has been developed. Different lipases have been tested in the transesterification of these tertiary alcohols; CAL-A (Candida antarctica Lipase A) was found to be the best biocatalyst for 1b and CAL-A-CLEA (Lipase A, C. antarctica, cross-linked enzyme aggregate) for 1a, obtained with ee values of 20% and 45%, respectively, and the corresponding esters 2b and 2a with the ee values of 99% and 71%, respectively.
Figure optionsDownload as PowerPoint slide
(R)-(−)-1-Methyl-2,3-dihydro-1H-inden-1-yl acetateC12H14O2[α]D26=-5.0 (c 0.25, CHCl3)ee = 71%Source of chirality: enzymatic resolutionAbsolute configuration: (R)
(R)-(−)-1-Methyl-1,2,3,4-tetrahydronaphthalen-1-yl-acetateC13H16O2[α]D26=-14.3 (c 0.60, CHCl3)ee = 99%Source of chirality: enzymatic resolutionAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 23, 1 December 2008, Pages 2717–2720