| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347726 | 980323 | 2008 | 6 صفحه PDF | دانلود رایگان |
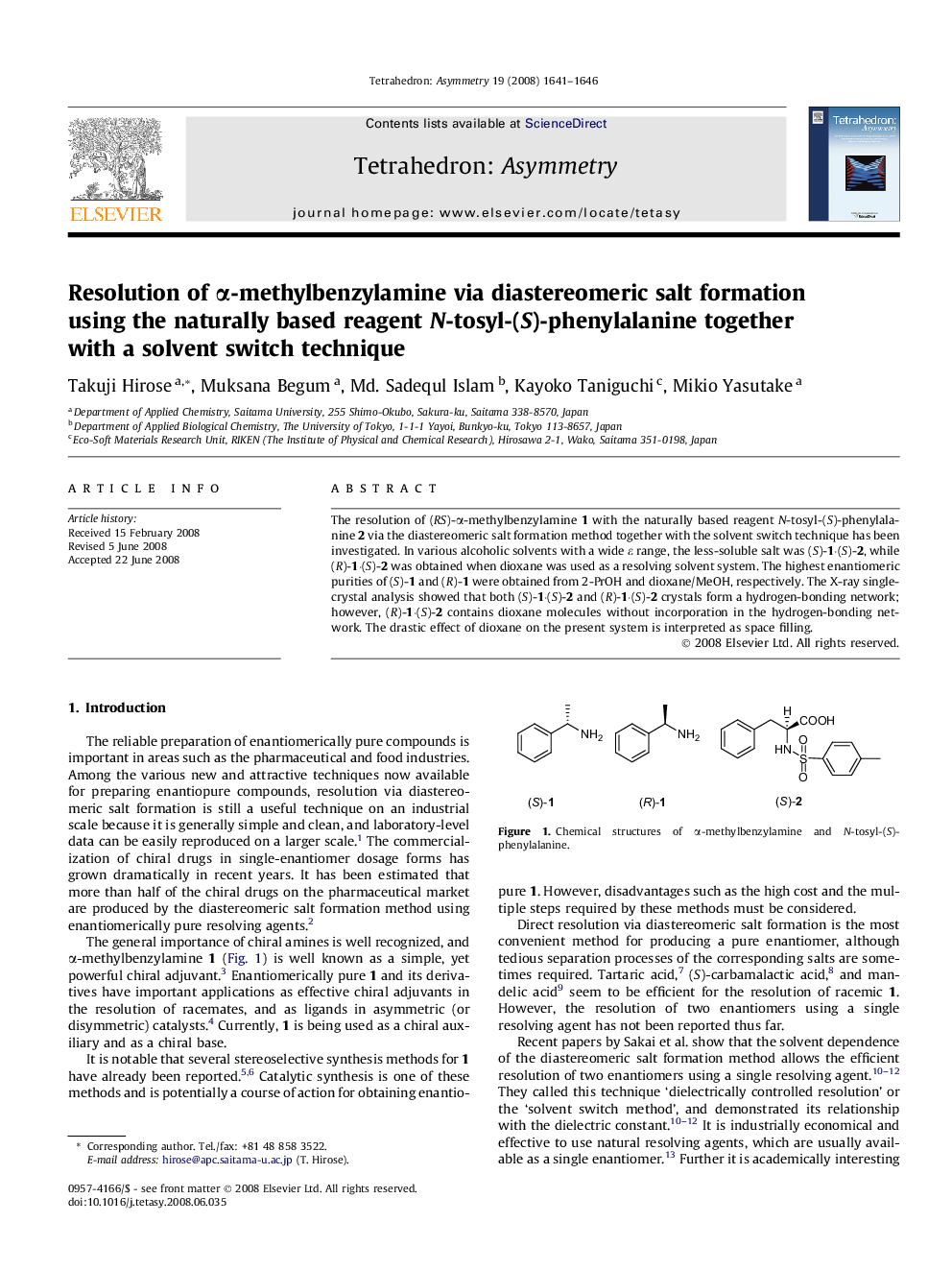
The resolution of (RS)-α-methylbenzylamine 1 with the naturally based reagent N-tosyl-(S)-phenylalanine 2 via the diastereomeric salt formation method together with the solvent switch technique has been investigated. In various alcoholic solvents with a wide ε range, the less-soluble salt was (S)-1·(S)-2, while (R)-1·(S)-2 was obtained when dioxane was used as a resolving solvent system. The highest enantiomeric purities of (S)-1 and (R)-1 were obtained from 2-PrOH and dioxane/MeOH, respectively. The X-ray single-crystal analysis showed that both (S)-1·(S)-2 and (R)-1·(S)-2 crystals form a hydrogen-bonding network; however, (R)-1·(S)-2 contains dioxane molecules without incorporation in the hydrogen-bonding network. The drastic effect of dioxane on the present system is interpreted as space filling.
Figure optionsDownload as PowerPoint slide
(S)-Methylbenzylamine·(S)-tosylphenylalanine saltC24H28N2O4S: 440.54[α]D27=+50.5 (c 0.107, MeOH)Absolute configuration: (S,S)
(R)-Methylbenzylamine·(S)-tosylphenylalanine saltC24H28N2O4S: 440.54[α]D23=+78.5 (c 0.107, MeOH)Absolute configuration: (R,S)
(R)-Methylbenzylamine·(S)-tosylphenylalanine·dioxane saltC28H36N2O6S: 528.65[α]D23=+20.7 (c 1.0, MeOH)Absolute configuration: (R,S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 14, 25 July 2008, Pages 1641–1646