| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347732 | 980323 | 2008 | 5 صفحه PDF | دانلود رایگان |
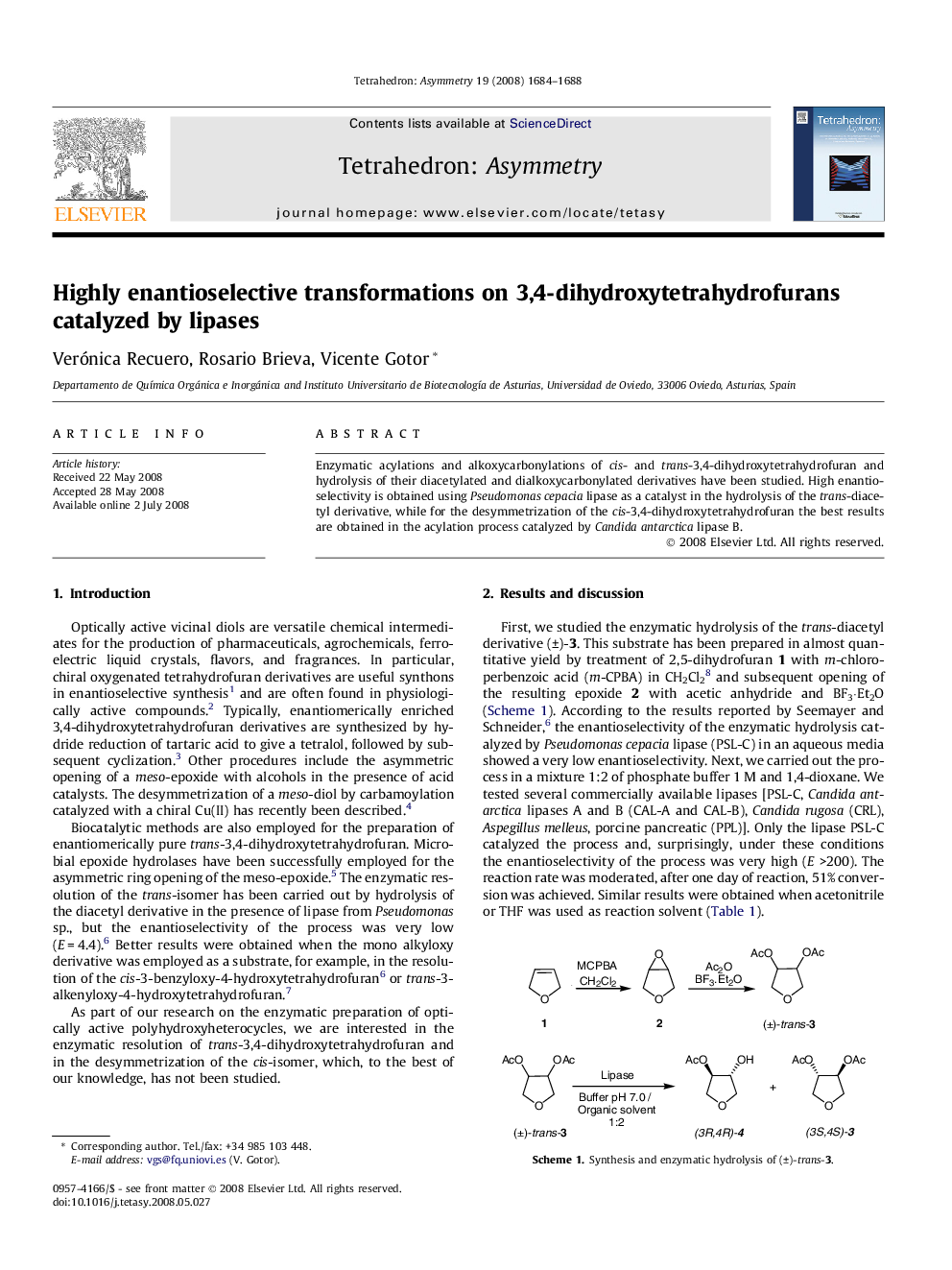
Enzymatic acylations and alkoxycarbonylations of cis- and trans-3,4-dihydroxytetrahydrofuran and hydrolysis of their diacetylated and dialkoxycarbonylated derivatives have been studied. High enantioselectivity is obtained using Pseudomonas cepacia lipase as a catalyst in the hydrolysis of the trans-diacetyl derivative, while for the desymmetrization of the cis-3,4-dihydroxytetrahydrofuran the best results are obtained in the acylation process catalyzed by Candida antarctica lipase B.
Figure optionsDownload as PowerPoint slide
(+)-3,4-DiacetoxytetrahydrofuraneC8H12O5Ee = >99%[α]D20=+20.4 (c 0.05, CHCl3)Source of chirality: lipase-catalyzed hydrolysisAbsolute configuration: (3S,4S)
(−)-3-Acetoxy-4-hydroxytetrahydrofuranC6H10O4Ee = 99%[α]D20=-6.0 (c 0.02, CHCl3)Source of chirality: lipase-catalyzed hydrolysisAbsolute configuration: (3R,4R)
(−)-3-Benzoyloxy-4-hydroxytetrahydrofuranC11H12O4Ee = 83%[α]D20=-12 (c 0.03, CHCl3)Source of chirality: lipase-catalyzed acylationAbsolute configuration: (3S,4R)
(−)-3-Ethoxycarbonyloxy-4-hydroxytetrahydrofuranC7H12O5Ee = 78%[α]D20=-4.6 (c 0.25, CHCl3)Source of chirality: lipase-catalyzed alcoxycarbonylationAbsolute configuration: (3S,4R)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 14, 25 July 2008, Pages 1684–1688