| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347739 | 980323 | 2008 | 14 صفحه PDF | دانلود رایگان |
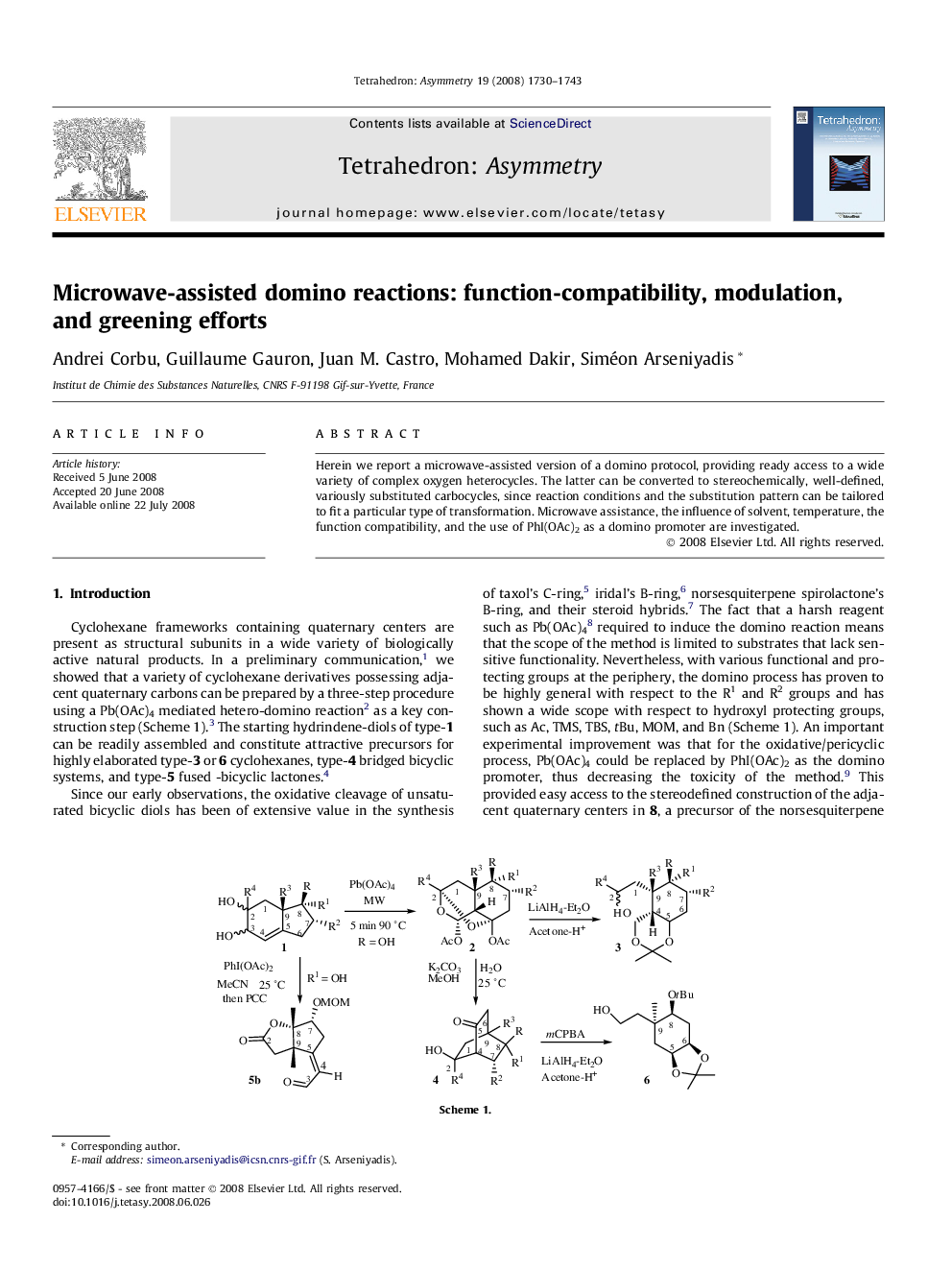
Herein we report a microwave-assisted version of a domino protocol, providing ready access to a wide variety of complex oxygen heterocycles. The latter can be converted to stereochemically, well-defined, variously substituted carbocycles, since reaction conditions and the substitution pattern can be tailored to fit a particular type of transformation. Microwave assistance, the influence of solvent, temperature, the function compatibility, and the use of PhI(OAc)2 as a domino promoter are investigated.
Figure optionsDownload as PowerPoint slide
(1S,2R,5R,6S,7aS)-2-(Methoxymethoxy)-1,7a-dimethyl-2,3,5,6,7,7a-hexahydro-1H-indene-1,5,6-triolC13H22O5Ee = >97%[α]D20=+18 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1S,2R,5R,6S,7aS)
(3S,3aS,5S,6R)-5-Allyl-3-tert-butoxy-3a-methyl-2,3,3a,4,5,6-hexahydro-1H-indene-5,6-diolC17H28O3Ee = >97%[α]D20=-7 (c 0.25, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (3S,3aS,5S,6R)
(3S,3aS,5S,6R)-3-tert-Butoxy-3a,5-dimethyl-2,3,3a,4,5,6-hexahydro-1H-indene-5,6-diolC15H26O3Ee = >97%[α]D20=-11 (c 0.7, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (3S,3aS,5S,6R)
(2R,3aS,7S,8S,91R)-2-Methyl-1,2,3a,4,5,7,8,9-octahydroindeno[1-b]furan-7,8-diolC12H18O3Ee = 81%[α]D20=+34 (c 0.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (2R,3aS,7S,8S,91R)
(5′S,6′S,7a′R)-7a′-((E)-Prop-1-enyl)-2′,3′,5′,6′,7′,7a′-hexahydrospiro[[1,3]dioxolane-2,1′-indene]-5′,6′-diolC14H20O4Ee = 81%[α]D20-61 (c 1.8, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (5′S,6′S,7a′R)
Acetic acid 3-acetoxy-1-allyl-6-tert-butoxy-7-methyl-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC21H32O7Ee = >97%[α]D20=-28 (c 0.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,6S,7S,8S,9R)
Acetic acid 3-acetoxy-6-tert-butoxy-1,7-dimethyl-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC19H30O7Ee = >97%[α]D20=-34 (c 0.9, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,6S,7S,8S,9R)
Acetic acid 3-acetoxy-6-(prop-1-enyl)-2,10-dioxa-spiro[[1,3]dioxolane-2′,6-tricyclo[5.3.1.00,0]undec]-9-yl esterC18H24O8Ee = 81%[α]D20=-28 (c 1.4, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (2R,3S,4S,5R,9S)
Acetic acid 3-acetoxy-6-hydroxy-6,7-dimethyl-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC15H22O7Ee = >97%[α]D20=-44 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,6S,7S,8S,9R)
Acetic acid 3-acetoxy-6,7-(1′-methylepoxyethano)-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC16H22O7Ee = 81%[α]D20=-29 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (2R,3S,4S,5R,8S,9S)
(1R,2S,4S,5S)-2-(tert-Butyldimethylsilyloxy)-5-(methoxymethoxy)-4,5-dimethylbicyclo[2.2.2]octan-7-oneC18H34O4SiEe = >97%[α]D20=+2 (c 1.4, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,2S,4S,5S)
(1R,2R,4S,5S)-2-(tert-Butyldimethylsilyloxy)-5-(methoxymethoxy)-4,5-dimethylbicyclo[2.2.2]octan-7-oneC18H34O4SiEe = >97%[α]D20=+5 (c 1.4, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,2R,4S,5S)
(3R,4aS,5S)-3-(tert-Butyldimethylsilyloxy)-5-(methoxymethoxy)-4a,5-dimethyl-4a,5,6,7-tetrahydro-3H-isochromen-8(4H)-oneC19H34O5SiEe = >97%[α]D20=+57 (c 2.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (3R,4aS,5S)
11-Hydroxy-3-methyl-4-oxa-tricyclo[5.2.2.00,0]undecan-8-oneC11H16O3Ee = 81%[α]D20=+26 (c 0.8, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1S,3R,5S,7R,11R)
(1R,2S,4S,5S)-5-(tert-Butyldimethylsilyloxy)-2-hydroxy-4-((E)-prop-1-enyl)bicyclo[2.2.2]octan-7-oneC17H30O3SiEe = 81%[α]D20=+24 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,2S,4S,5S)
(1R,2R,4S,5S)-5-(tert-Butyldimethylsilyloxy)-2-hydroxy-4-((E)-prop-1-enyl)bicyclo[2.2.2]octan-7-oneC17H30O3SiEe = 81%[α]D20=+38 (c 1.8, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,2R,4S,5S)
(1R,2R,4S)-2-Hydroxy-4-methyl-5-methylenebicyclo[2.2.2]octan-7-oneC10H14O2Ee = >97%[α]D20=+19 (c 0.8, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,2R,4S)
(1R,2S,4S)-2-Hydroxy-4-methyl-5-methylenebicyclo[2.2.2]octan-7-oneC10H14O2Ee = >97%[α]D20=+10 (c 0.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,2S,4S)
(Z)-2-((3aS,6R,6aS)-6-(Methoxymethoxy)-3a,6a-dimethyl-2-oxodihydro-2H-cyclopenta[b]furan-4(5H,6H,6aH)-ylidene)acetaldehydeC13H18O5Ee = >97%[α]D20=-101 (c 0.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (3aS,6R,6aS)
(3aR,5R,6S)-3a-Methyl-3-methylene-2,3,3a,4,5,6-hexahydro-1H-indene-5,6-diolC11H16O2Ee = >97%[α]D20=+22 (c 1.3, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (3aR,5R,6S)
(2R,3aS,5R,6R)-5,6-Dihydroxy-3a-methyl-3-methylene-2,3,3a,4,5,6-hexahydro-1H-inden-2-yl acetateC13H18O4Ee = >97%[α]D20=-34 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (2R,3aS,5R,6R)
(2R,3aS,5R,6R)-2-(Methoxymethoxy)-3a-methyl-3-methylene-2,3,3a,4,5,6-hexahydro-1H-indene-5,6-diolC13H20O4Ee = >97%[α]D20=-36 (c 1.1, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (2R,3aS,5R,6R)
(E)-Ethyl 2-((5R,6S,7aS)-5,6-dihydroxy-7a-methyl-2,3,5,6,7,7a-hexahydro-1H-inden-1-ylidene)acetateC14H20O4Ee = >97%[α]D20=-8 (c 1.2, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (5R,6S,7aS)
(2R,3aS,91R)-2-Methyl-1,2,4,5,8,9-hexahydroindeno[1-b]furan-7(3aH)-oneC12H16O2Ee = 81%[α]D20=+163 (c 1.8, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (2R,3aS,91R)
(R,E)-7a′-(Prop-1-enyl)-2′,3′,7′,7a′-tetrahydrospiro[[1,3]dioxolane-2,1′-inden]-5′(6′H)-oneC14H18O3Ee = 81%[α]D20=+27 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (7a′R)
(6′R,7a′R)-5′-Oxo-7a′-((E)-prop-1-enyl)-2′,3′,5′,6′,7′,7a′-hexahydrospiro[[1,3]dioxolane-2,1′-indene]-6′-yl acetateC16H20O5Ee = 81%[α]D20=+59 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (6′R), (7a′R)
Acetic acid 3-acetoxy-7-methyl-6-oxo-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC14H18O7Ee = >97%[α]D20=+2 (c 0.6, CHCl3)Mp: 110–120 °CSource of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,7S,8S,9R)
Acetic acid 3-acetoxy-7-methyl-2,10-dioxa-spiro[[1,3]dioxolane-2′,6-tricylo[5.3.1.00,0]undec]-9-yl esterC16H22O8Ee = >97%[α]D20=-40 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (2R,3S,4S,5R,9S)
Acetic acid 3-acetoxy-7-methyl-6-methylene-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC15H20O6Ee = >97%[α]D20=-28 (c 1.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,7S,8S,9R)
Acetic acid 3,5-diacetoxy-7-methyl-6-methylene-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC17H22O8Ee = >97%[α]D20=-9 (c 1.0, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,5R,7S,8S,9R)
Acetic acid 3-acetoxy-5-methoxymethoxy-7-methyl-6-methylene-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC17H24O8Ee = >97%[α]D20=+14 (c 0.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,5R,7S,8S,9R)
(3,9-Diacetoxy-7-methyl-2,10-dioxa-tricyclo[5.3.1.00,0]undec-6-ylidene)-acetic acid ethyl esterC18H24O8Ee = >97%[α]D20=+36 (c 0.7, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3S,7S,8S,9R)
Acetic acid 3-acetoxy-6-tert-butoxy-7-methyl-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC18H28O7Ee = >97%[α]D20=+40 (c 1.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3R,6S,7S,8S,9R)
Acetic acid 3-acetoxy-7-methyl-6-oxo-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC14H18O7Ee = >97%[α]D20=+17 (c 1.2, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3R,7S,8S,9R)
Acetic acid 3-acetoxy-6-(tert-butyl-dimethyl-silanyloxy)-7-methyl-2,10-dioxa-tricyclo[5.3.1.00,0]undec-9-yl esterC14H18O7Ee = >97%[α]D20=+3 (c 1.5, CHCl3)Source of chirality: (S)-(−)-prolineAbsolute configuration: (1R,3R,8S,9R,6S,7S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 14, 25 July 2008, Pages 1730–1743