| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347807 | 1500354 | 2013 | 13 صفحه PDF | دانلود رایگان |
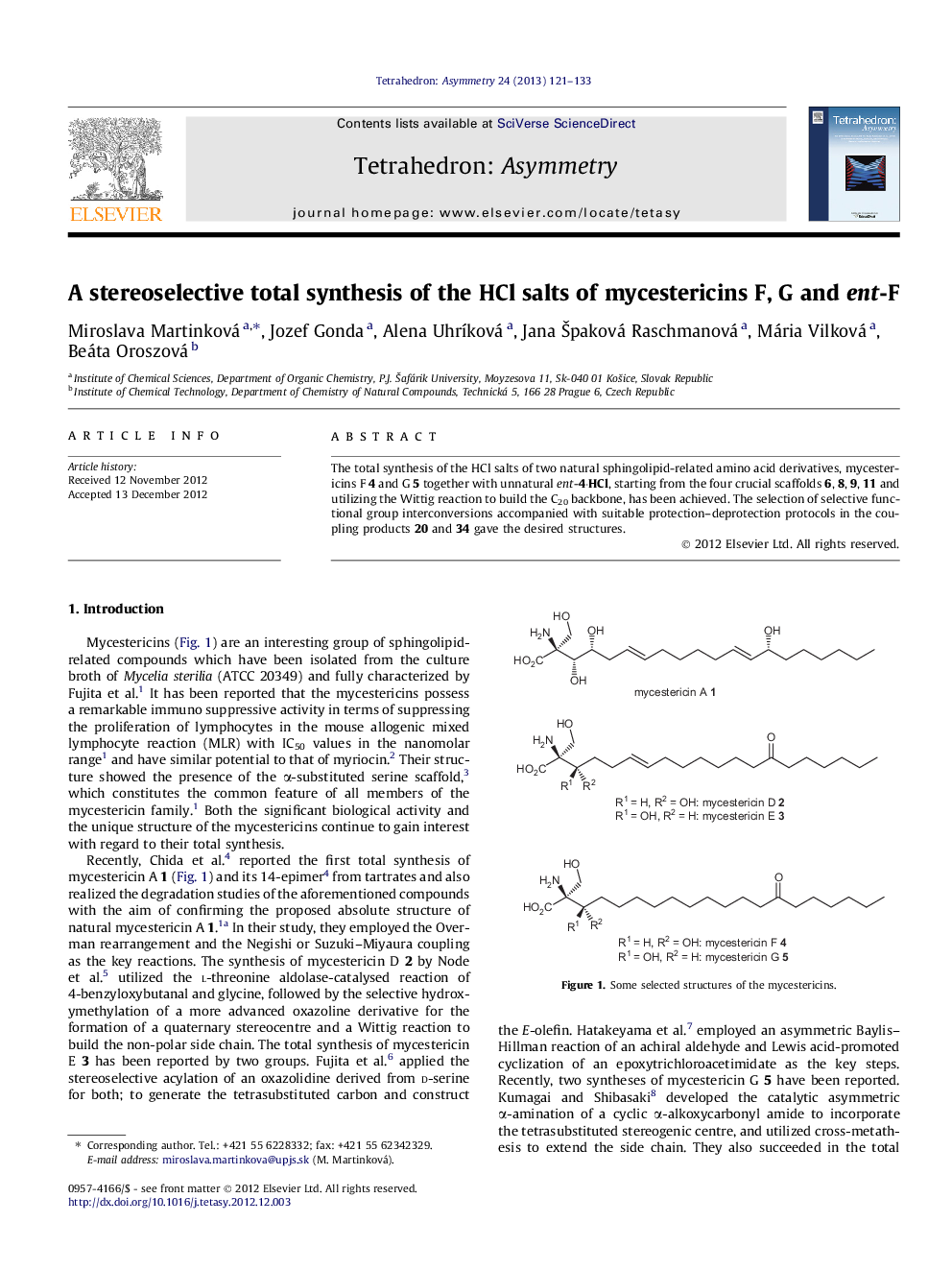
The total synthesis of the HCl salts of two natural sphingolipid-related amino acid derivatives, mycestericins F 4 and G 5 together with unnatural ent-4·HCl, starting from the four crucial scaffolds 6, 8, 9, 11 and utilizing the Wittig reaction to build the C20 backbone, has been achieved. The selection of selective functional group interconversions accompanied with suitable protection–deprotection protocols in the coupling products 20 and 34 gave the desired structures.
Figure optionsDownload as PowerPoint slide
Ethyl (4S)-4-{(4′R)-3′-benzyl-4′-[(1″S)-1″,2″-dihydroxyethyl]-2′-oxooxazolidin-4′-yl}-4-hydroxybutanoateC18H25NO7[α]D20=-23.1 (c 0.76, CHCl3)Source of chirality: d-xyloseAbsolute configuration: (1″S,4′R,4S)
3-[(5′R,6′S)-1′-Benzyl-8′,8′-dimethyl-2′-oxo-3′,7′,9′-trioxa-1′-azaspiro[4′.5′]decan-6′-yl]propanalC18H23NO5[α]D20=+61.6 (c 0.35, CHCl3)Source of chirality: d-xyloseAbsolute configuration: (5′R,6′S)
tert-Butyl {(4S,5S)-4-[10′-(2″-hexyl-1″,3″-dioxolan-2″-yl)decyl]-5-(hydroxymethyl)-2,2-dimethyl-1,3-dioxan-5-yl}carbamateC31H59NO7[α]D20=+22.2 (c 0.52, CHCl3)Source of chirality: d-xyloseAbsolute configuration: (4S,5S)
(2S,3S)-2-Amino-3-hydroxy-2-(hydroxymethyl)-14-oxoicosanoic acid hydrochlorideC21H42ClNO5[α]D20=-3.5 (c 0.18, CH3OH)Source of chirality: d-xyloseAbsolute configuration: (2S,3S)
(2R,3R)-2-Amino-3-hydroxy-2-(hydroxymethyl)-14-oxoicosanoic acid hydrochlorideC21H42ClNO5[α]D20=+4.4 (c 0.16, CH3OH)Source of chirality: d-xyloseAbsolute configuration: (2R,3R)
(4S)-4-[(1′R)-1′-Hydroxy-12′-oxooctadecyl]-4-(hydroxymethyl)oxazolidin-2-oneC22H40NO5[α]D20=+9.3 (c 0.54, CHCl3)Source of chirality: d-xyloseAbsolute configuration: (1′R,4S)
(2S,3R)-2-Amino-3-hydroxy-2-(hydroxymethyl)-14-oxoicosanoic acid hydrochlorideC21H42ClNO5[α]D20=+10.5 (c 0.24, CH3OH)Source of chirality: d-xyloseAbsolute configuration: (2S,3R)
Journal: Tetrahedron: Asymmetry - Volume 24, Issues 2–3, 15 February 2013, Pages 121–133