| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347837 | 980327 | 2007 | 7 صفحه PDF | دانلود رایگان |
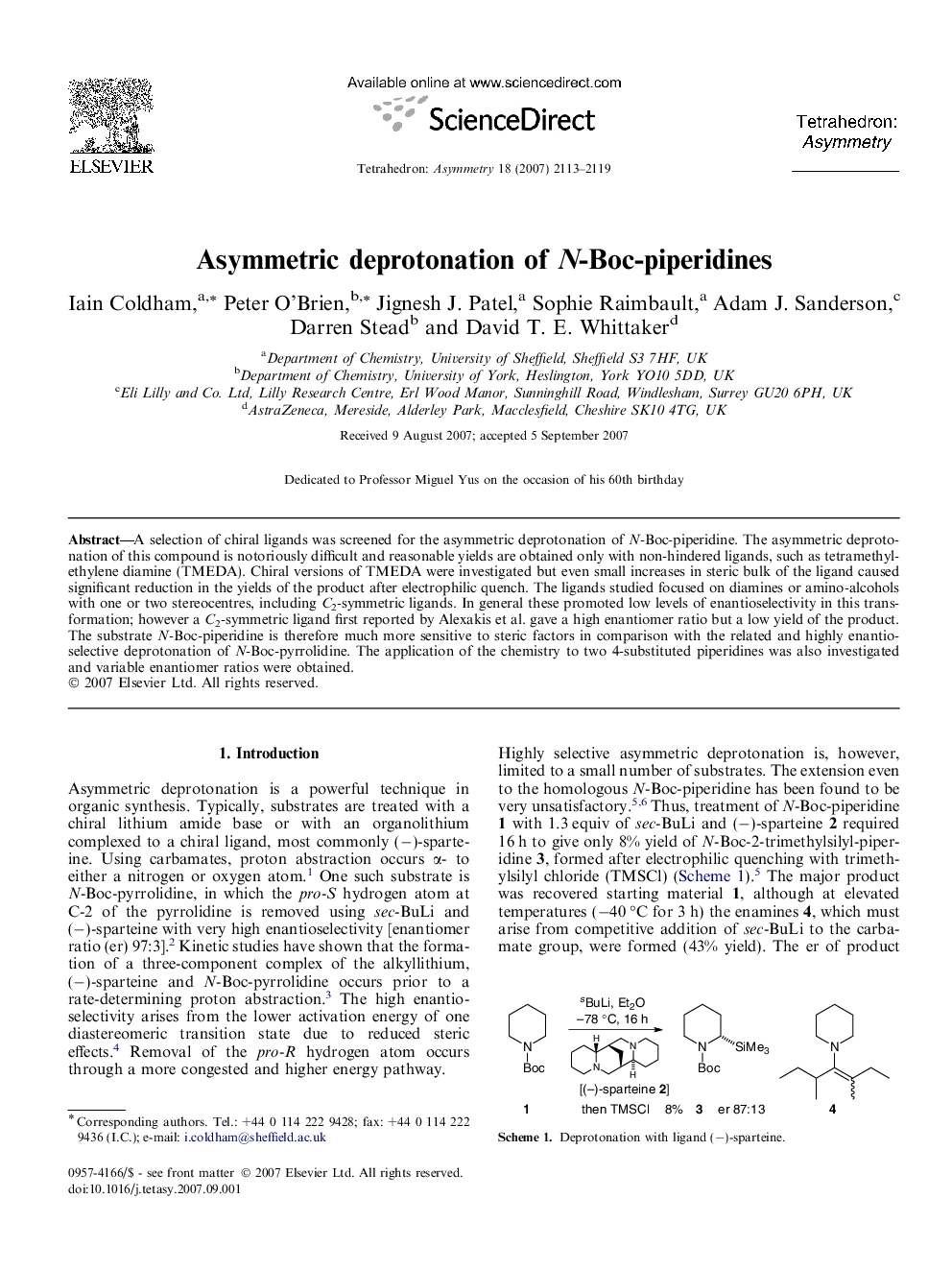
A selection of chiral ligands was screened for the asymmetric deprotonation of N-Boc-piperidine. The asymmetric deprotonation of this compound is notoriously difficult and reasonable yields are obtained only with non-hindered ligands, such as tetramethylethylene diamine (TMEDA). Chiral versions of TMEDA were investigated but even small increases in steric bulk of the ligand caused significant reduction in the yields of the product after electrophilic quench. The ligands studied focused on diamines or amino-alcohols with one or two stereocentres, including C2-symmetric ligands. In general these promoted low levels of enantioselectivity in this transformation; however a C2-symmetric ligand first reported by Alexakis et al. gave a high enantiomer ratio but a low yield of the product. The substrate N-Boc-piperidine is therefore much more sensitive to steric factors in comparison with the related and highly enantioselective deprotonation of N-Boc-pyrrolidine. The application of the chemistry to two 4-substituted piperidines was also investigated and variable enantiomer ratios were obtained.
Figure optionsDownload as PowerPoint slide
(S)-N-tert-Butoxycarbonyl-2-trimethylsilylpiperidineC13H27NO2Si[α]D24=+17.5 (c 0.7, CHCl3)Source of chirality: asymmetric deprotonationAbsolute configuration: (S)
(S)-N,N,N′,N′-Tetramethyl-3-phenylpropane-1,2-diamineC13H23N2[α]D24=+50.0 (c 1.6, CHCl3)Source of chirality: l-phenylalanineAbsolute configuration: (S)
(S)-N,N,N′,N′-Tetramethyl-1,2-diamino-3-methylbutaneC9H23N2[α]D24=+28.0 (c 2.5, CHCl3)Source of chirality: l-valineAbsolute configuration: (S)
(S)-N1-Ethyl-N,N′,N′-trimethyl-3-methylbutane-1,2-diamineC10H25N2[α]D24=+26.0 (c 3.1, CHCl3)Source of chirality: l-valineAbsolute configuration: (S)
(S)-N1-Isobutyl-N,N′,N′-trimethyl-3-methylbutane-1,2-diamineC12H28N2[α]D24=+11.8 (c 2.8, CHCl3)Source of chirality: l-valineAbsolute configuration: (S)
(S)-N1-(2-Dimethylaminoethyl)-N,N′,N′-trimethyl-3-methylbutane-1,2-diamineC12H30N3[α]D24=+23.8 (c 2.2, CHCl3)Source of chirality: l-valineAbsolute configuration: (S)
(S)-N,N,N′-Trimethyl-N′-(1-phenylethyl)ethane-1,2-diamineC13H22N2[α]D24=-33.1 (c 2.6, CHCl3)Source of chirality: (S)-1-phenylethylamineAbsolute configuration: (S)
(S,S)-N-tert-Butoxycarbonyl-4-phenyl-2-trimethylsilylpiperidineC19H32NO2Si[α]D24=+4.6 (c 1.1, CHCl3)Source of chirality: asymmetric deprotonationAbsolute configuration: (S,S)
(S)-N-tert-Butoxycarbonyl-4,4-dioxolanyl-2-trimethylsilylpiperidineC15H30NO4Si[α]D24=-0.5 (c 1.0, CHCl3)Source of chirality: asymmetric deprotonationAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 17, 4 September 2007, Pages 2113–2119