| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347863 | 980328 | 2008 | 6 صفحه PDF | دانلود رایگان |
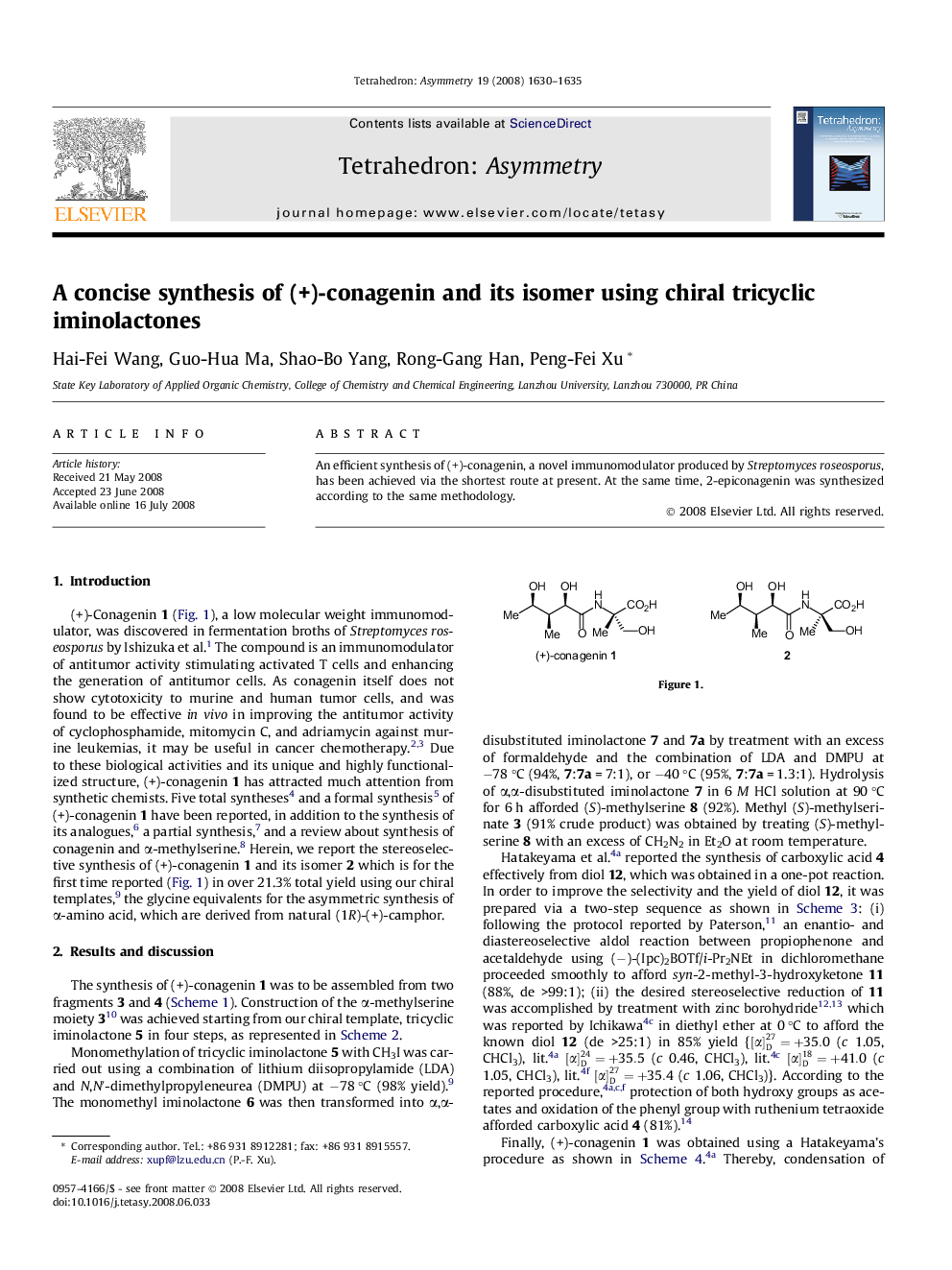
An efficient synthesis of (+)-conagenin, a novel immunomodulator produced by Streptomyces roseosporus, has been achieved via the shortest route at present. At the same time, 2-epiconagenin was synthesized according to the same methodology.
Figure optionsDownload as PowerPoint slide
Methyl N-[(2R,3S,4R)-2,4-diacetyloxy-3-methylvaleryl]-2-methyl-l-serinateC15H25NO8[α]D15=+34.0 (c 0.48, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3S,4R,S)
Methyl N-[(2R,3S,4R)-2,4-diacetyloxy-3-methylvaleryl]-2-methyl-d-serinateC15H25NO8[α]D15=+13.0 (c 0.62, CHCl3)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3S,4R,R)
(+)-ConageninC10H19NO6[α]D23=+50.0 (c 0.45, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3S,4R,S)
2-epi-ConageninC10H19NO6[α]D23=+24.0 (c 0.51, MeOH)Source of chirality: asymmetric synthesisAbsolute configuration: (2R,3S,4R,R)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 13, 11 July 2008, Pages 1630–1635