| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347958 | 980332 | 2008 | 4 صفحه PDF | دانلود رایگان |
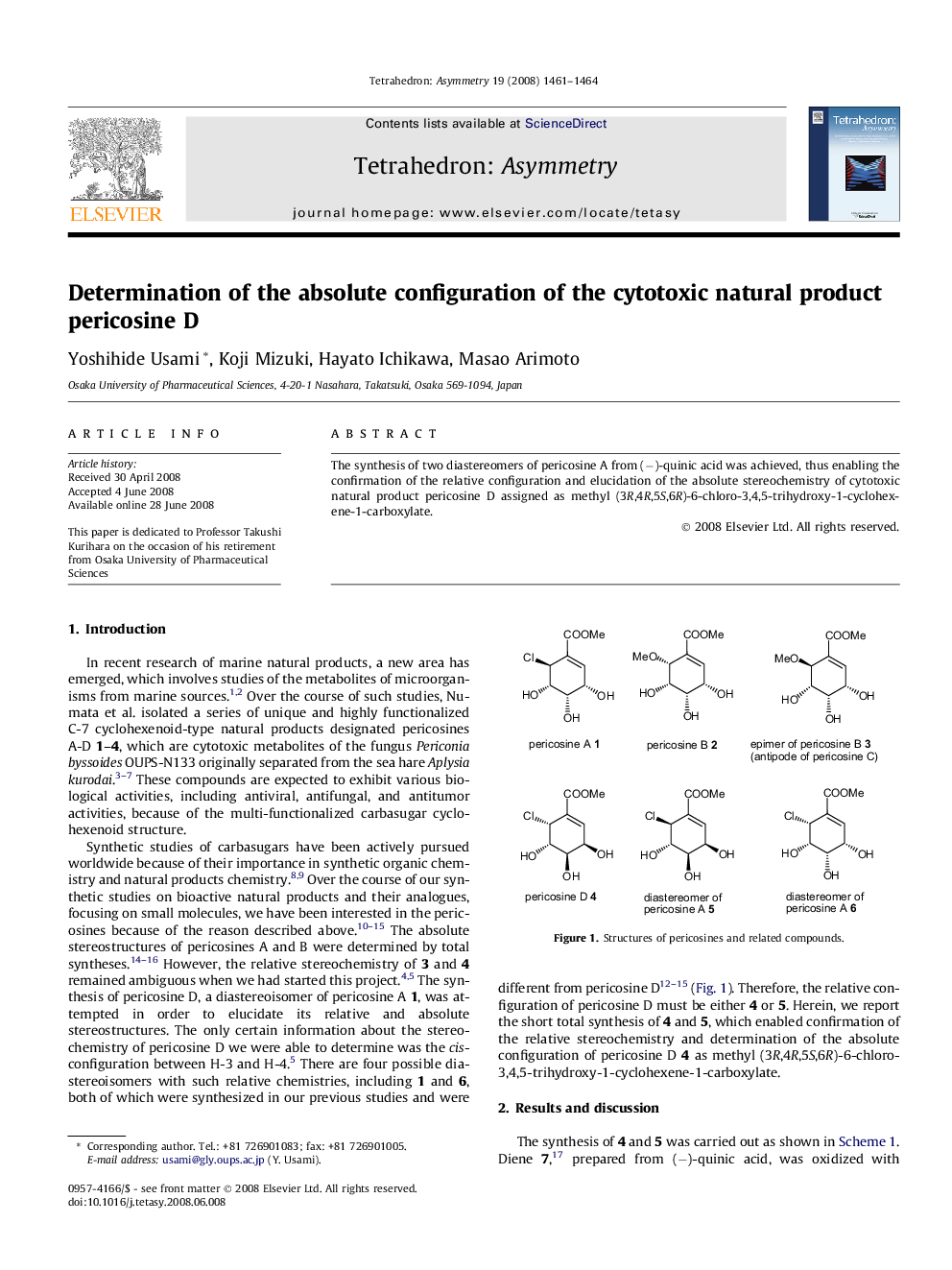
The synthesis of two diastereomers of pericosine A from (−)-quinic acid was achieved, thus enabling the confirmation of the relative configuration and elucidation of the absolute stereochemistry of cytotoxic natural product pericosine D assigned as methyl (3R,4R,5S,6R)-6-chloro-3,4,5-trihydroxy-1-cyclohexene-1-carboxylate.
Figure optionsDownload as PowerPoint slide
Methyl (3R,4R,5S,6S)-6-chloro-3,4,5-trihydroxy-1-cyclohexene-1-carboxylateC8H11O5Cl[α]D25=-101.0 (c 0.20, EtOH)Chiral source: synthesis from (−)-quinic acidAbsolute configuration: (3R,4R,5S,6S)
Methyl (3R,4R,5S,6R)-6-chloro-3,4,5-trihydroxy-1-cyclohexene-1-carboxylate, (−)-pericosine DC8H11O5Cl[α]D25=-275.4 (c 0.04, EtOH)Chiral source: synthesis from (−)-quinic acidAbsolute configuration: (3R,4R,5S,6R)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 12, 30 June 2008, Pages 1461–1464