| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1347964 | 980332 | 2008 | 5 صفحه PDF | دانلود رایگان |
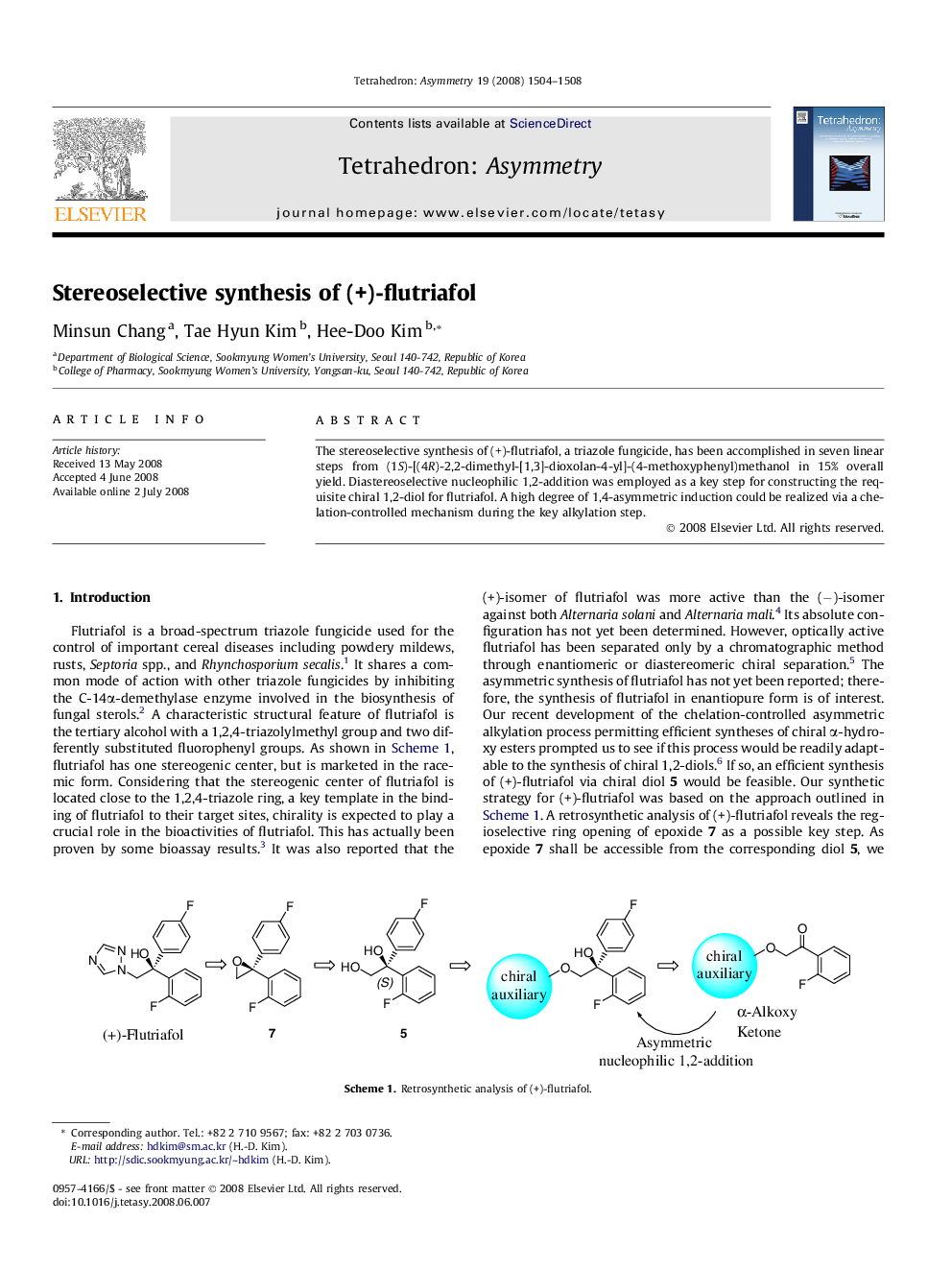
The stereoselective synthesis of (+)-flutriafol, a triazole fungicide, has been accomplished in seven linear steps from (1S)-[(4R)-2,2-dimethyl-[1,3]-dioxolan-4-yl]-(4-methoxyphenyl)methanol in 15% overall yield. Diastereoselective nucleophilic 1,2-addition was employed as a key step for constructing the requisite chiral 1,2-diol for flutriafol. A high degree of 1,4-asymmetric induction could be realized via a chelation-controlled mechanism during the key alkylation step.
Figure optionsDownload as PowerPoint slide
2-{[(R)-[(4R)-2,2-Dimethyl-1,3-dioxolan-4-yl](4-methoxyphenyl)methyl]oxy}-1-(2-fluorophenyl)ethan-1-oneC21H23FO5Ee = 99%[α]D24=-68.6 (c 0.11, CHCl3)Source of chirality: d-mannitol and stereoselective synthesisAbsolute configuration: (1′R,4″R)
2-{[(R)-[(4R)-2,2-Dimethyl-1,3-dioxolan-4-yl](4-methoxyphenyl)methyl]oxy}-(1S)-1-(2-fluorophenyl)-1-(4-fluorophenyl)ethanolC27H28F2O5Ee = 99%[α]D23=-32.9 (c 0.95, CHCl3)Source of chirality: d-mannitol and stereoselective synthesisAbsolute configuration: (1S,1′R,4″R)
2-{[(R)-[(4R)-2,2-Dimethyl-1,3-dioxolan-4-yl](4-methoxyphenyl)methyl]oxy}-(1R)-1-(2-fluorophenyl)-1-(4-fluorophenyl)ethanolC27H28F2O5Ee = 99%[α]D23=-28.2 (c 0.66, CHCl3)Source of chirality: d-mannitol and stereoselective synthesisAbsolute configuration: (1R,1′R,4″R)
(S)-1-(2-Fluorophenyl)-1-(4-fluorophenyl)ethane-1,2-diolC14H12F2O2Ee = 96%[α]D22=-25.6 (c 0.8, CHCl3)Source of chirality: d-mannitol and stereoselective synthesisAbsolute configuration: (S)
(S)-Toluene-4-sulfonic acid 2-(4-fluorophenyl)-2-(2-fluorophenyl)-2-hydroxy ethyl esterC21H18F2O4SEe = 96%[α]D24=-26.7 (c 0.12, CHCl3)Source of chirality: d-mannitol and stereoselective synthesisAbsolute configuration: (S)
(S)-(+)-FlutriafolC16H13F2N3OEe = 96%[α]D23=+21.8 (c 0.2, CHCl3)Source of chirality: d-mannitol and stereoselective synthesisAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 12, 30 June 2008, Pages 1504–1508