| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348060 | 980337 | 2006 | 5 صفحه PDF | دانلود رایگان |
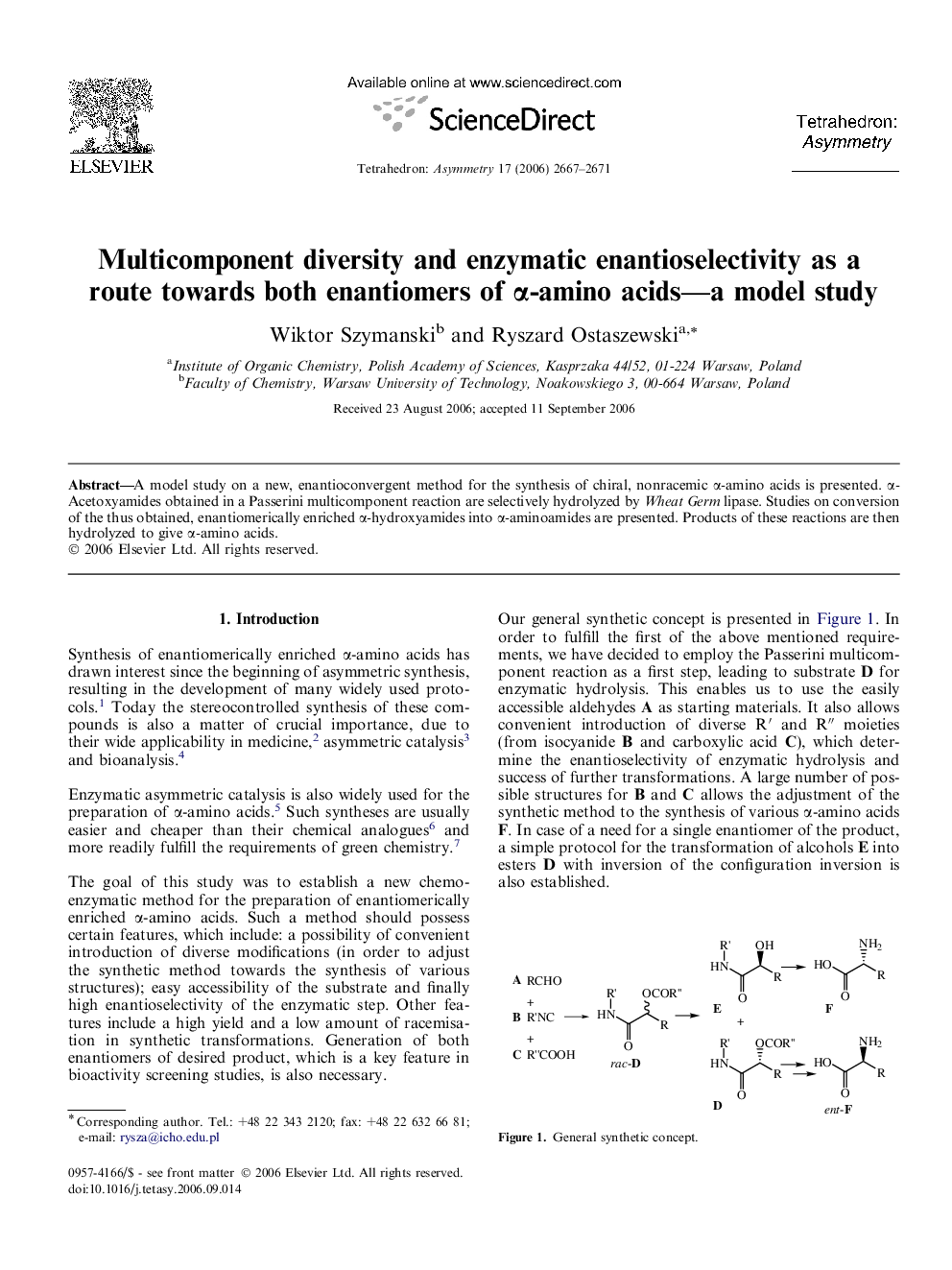
A model study on a new, enantioconvergent method for the synthesis of chiral, nonracemic α-amino acids is presented. α-Acetoxyamides obtained in a Passerini multicomponent reaction are selectively hydrolyzed by Wheat Germ lipase. Studies on conversion of the thus obtained, enantiomerically enriched α-hydroxyamides into α-aminoamides are presented. Products of these reactions are then hydrolyzed to give α-amino acids.
Figure optionsDownload as PowerPoint slide
(S)-2-Hydroxy-N-(4-methoxy-benzyl)-3-phenyl-propionamideC17H19NO3[α]D25=-71.1 (c 1.0, chloroform)Chirality source: stereocontrolled synthesis from commercially available enantiopure compoundAbsolute configuration: (S)
Methanesulfonic acid (S)-1-(4-methoxy-benzylcarba-moyl)-2-phenyl-ethyl esterC18H21NO5S[α]D25=-66.0 (c 1.00, chloroform)Chirality source: stereocontrolled synthesis from commercially available enantiopure compoundAbsolute configuration: (S)
(R)-2-Azido-N-(4-methoxy-benzyl)-3-phenyl-propionamideC17H18N4O2[α]D25=-21.8 (c 1.00, chloroform)Chirality source: stereocontrolled synthesis from commercially available enantiopure compoundAbsolute configuration: (S)
(R)-PhenylalanineC9H11NO2[α]D25=-7.3 (c 1.00, acetic acid)Chirality source: stereocontrolled synthesis from commercially available enantiopure compoundAbsolute configuration: (S)
Toluene-4-sulfonic acid (S)-1-(4-methoxy-benzylcarbamoyl)-2-phenyl-ethyl esterC24H25NO5S[α]D25=-50.4 (c 1.0, chloroform)Chirality source: stereocontrolled synthesis from commercially available enantiopure compoundAbsolute configuration: (S)
Acetic acid (S)-1-(4-methoxy-benzylcarbamoyl)-2-phenyl-ethyl esterC19H21NO4[α]D25=-10.9 (c 1.0, chloroform)Chirality source: stereocontrolled synthesis from commercially available enantiopure compoundAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 17, Issue 18, 16 October 2006, Pages 2667–2671