| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348202 | 980344 | 2011 | 7 صفحه PDF | دانلود رایگان |
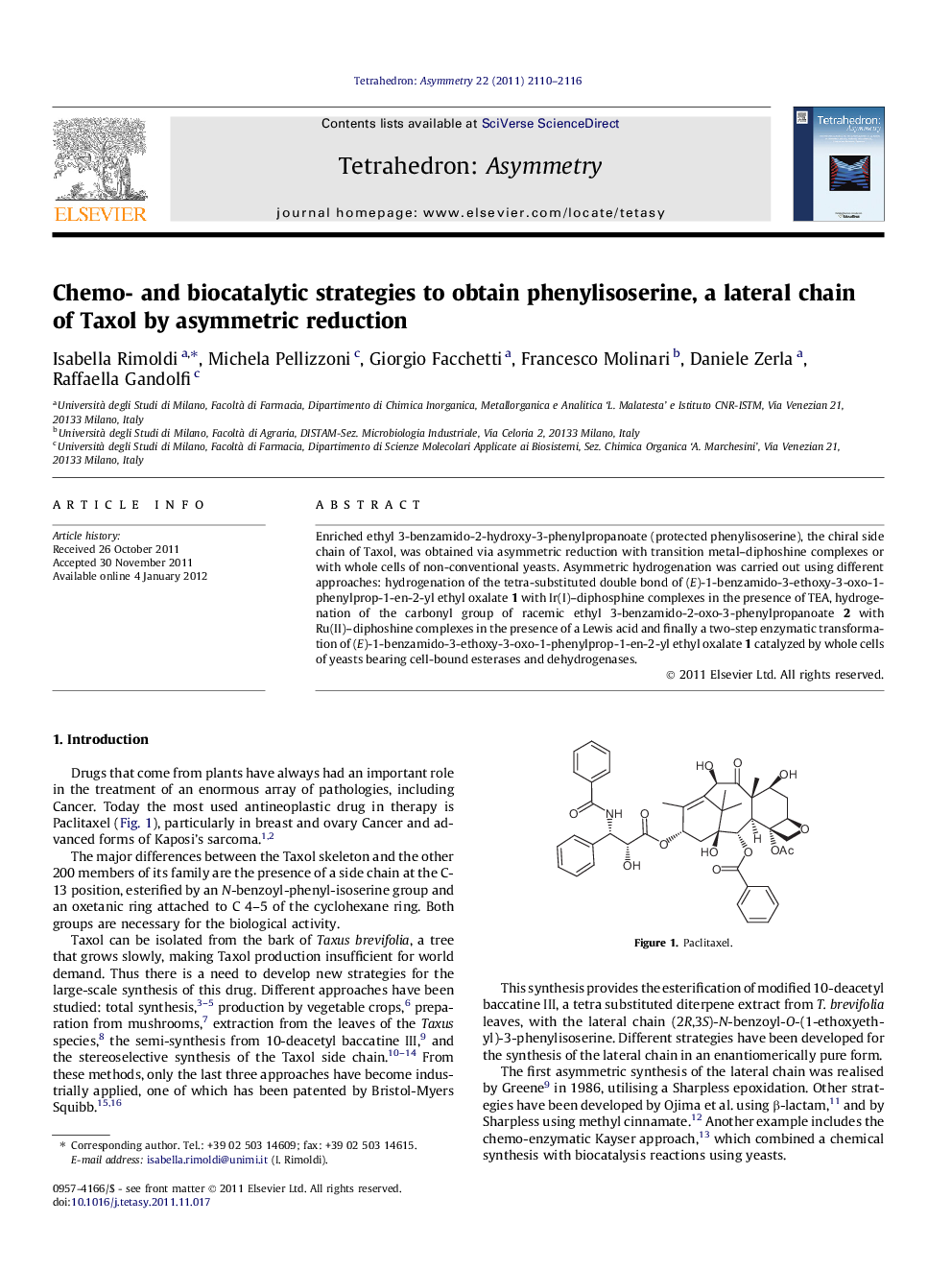
Enriched ethyl 3-benzamido-2-hydroxy-3-phenylpropanoate (protected phenylisoserine), the chiral side chain of Taxol, was obtained via asymmetric reduction with transition metal–diphoshine complexes or with whole cells of non-conventional yeasts. Asymmetric hydrogenation was carried out using different approaches: hydrogenation of the tetra-substituted double bond of (E)-1-benzamido-3-ethoxy-3-oxo-1-phenylprop-1-en-2-yl ethyl oxalate 1 with Ir(I)–diphosphine complexes in the presence of TEA, hydrogenation of the carbonyl group of racemic ethyl 3-benzamido-2-oxo-3-phenylpropanoate 2 with Ru(II)–diphoshine complexes in the presence of a Lewis acid and finally a two-step enzymatic transformation of (E)-1-benzamido-3-ethoxy-3-oxo-1-phenylprop-1-en-2-yl ethyl oxalate 1 catalyzed by whole cells of yeasts bearing cell-bound esterases and dehydrogenases.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 24, 31 December 2011, Pages 2110–2116