| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348203 | 980344 | 2011 | 7 صفحه PDF | دانلود رایگان |
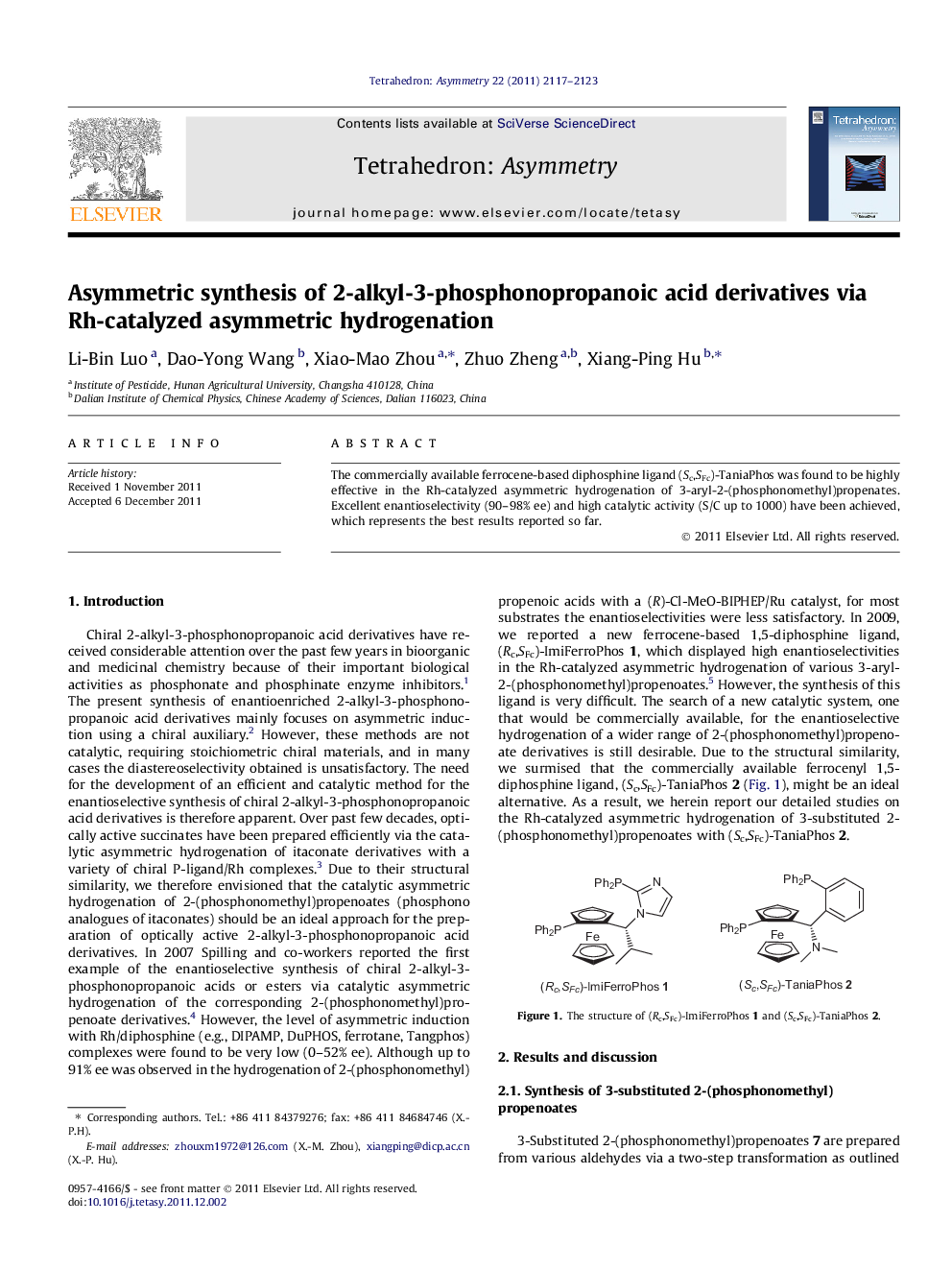
The commercially available ferrocene-based diphosphine ligand (Sc,SFc)-TaniaPhos was found to be highly effective in the Rh-catalyzed asymmetric hydrogenation of 3-aryl-2-(phosphonomethyl)propenates. Excellent enantioselectivity (90–98% ee) and high catalytic activity (S/C up to 1000) have been achieved, which represents the best results reported so far.
Figure optionsDownload as PowerPoint slide
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-phenyl-2-propionateC14H21O5Pee = 96%[α]D20=+16.8 (c 1.3, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(4-fluorophenyl)-2-propionateC14H20FO5Pee = 91%[α]D20=+17.9 (c 1.4, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(4-chlorophenyl)-2-propionateC14H20ClO5Pee = 96%[α]D20=+21.1 (c 1.2, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(4-nitrophenyl)-2-propionateC14H20NO7Pee = 95%[α]D20=+23.7 (c 1.1, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(4-methoxyphenyl)-2-propionateC15H23O6Pee = 95%[α]D20=+21.5 (c 1.2, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(3-methoxyphenyl)-2-propionateC15H23O6Pee = 95%[α]D20=+18.8 (c 1.2, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(2-methoxyphenyl)-2-propionateC15H23O6Pee = 97%[α]D20=+27.9 (c 1.1, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(3-chlorophenyl)-2-propionateC14H20ClO5Pee = 92%[α]D20=+17.1 (c 1.2, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(2-furyl)-2-propionateC12H19O6Pee = 90%[α]D20=+10.6 (c 1.1, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Ethyl 2-[(dimethoxyphosphoryl)methyl]-3-(2-thienyl)-2-propionateC12H19O5PSee = 98%[α]D20=+13.1 (c 1.2, CHCl3)Source of chirality: asymmetric catalysisAbsolute configuration: n.d.
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 24, 31 December 2011, Pages 2117–2123