| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348205 | 980344 | 2011 | 10 صفحه PDF | دانلود رایگان |
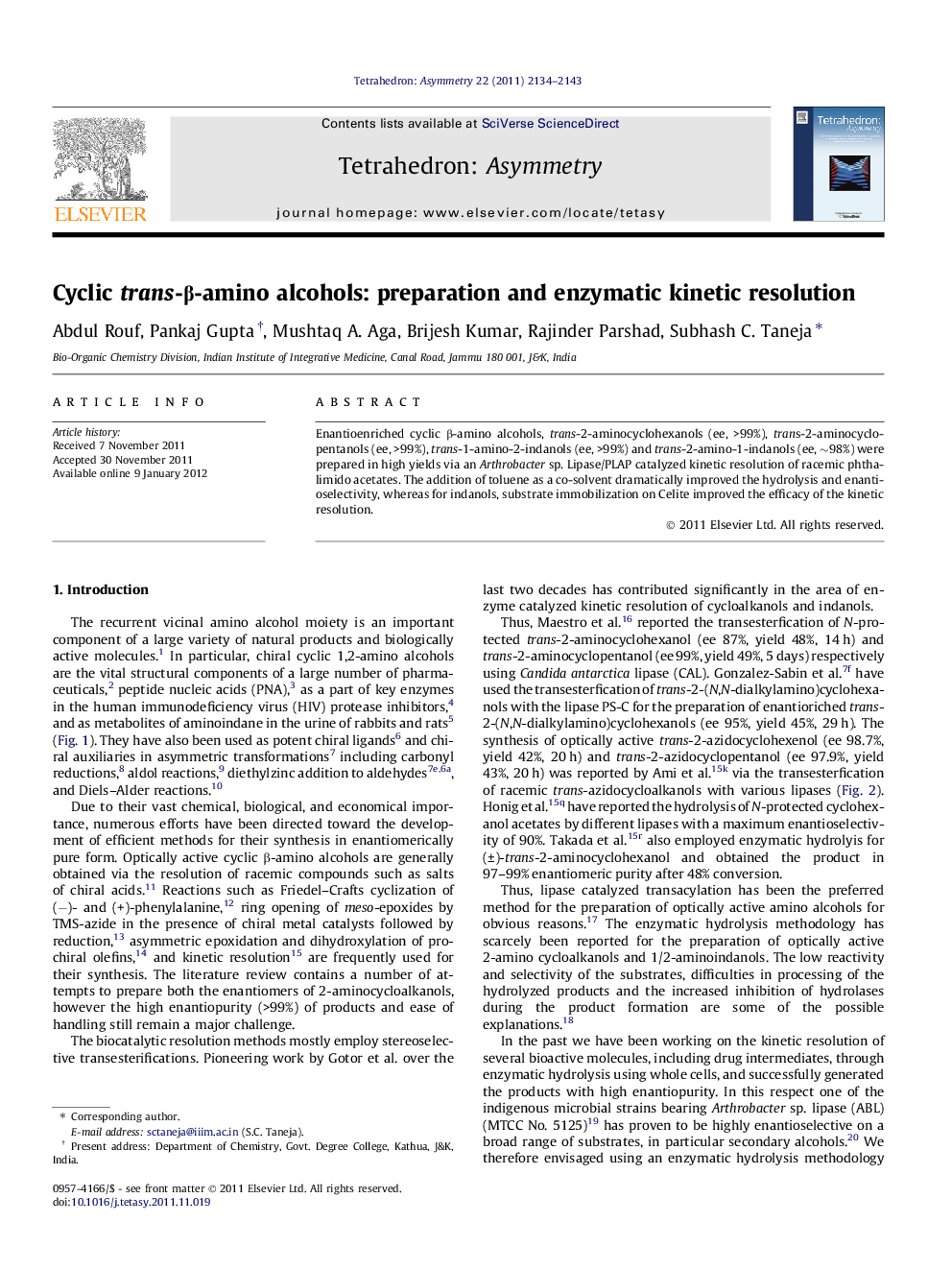
Enantioenriched cyclic β-amino alcohols, trans-2-aminocyclohexanols (ee, >99%), trans-2-aminocyclopentanols (ee, >99%), trans-1-amino-2-indanols (ee, >99%) and trans-2-amino-1-indanols (ee, ∼98%) were prepared in high yields via an Arthrobacter sp. Lipase/PLAP catalyzed kinetic resolution of racemic phthalimido acetates. The addition of toluene as a co-solvent dramatically improved the hydrolysis and enantioselectivity, whereas for indanols, substrate immobilization on Celite improved the efficacy of the kinetic resolution.
Figure optionsDownload as PowerPoint slide
(1R,2R)-(−)-2-Phthalimidocyclopentan-1-olC13H13NO3ee >99%[α]D25=-32.5 (c 1, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1R,2R)
(1S,2S)-(+)-2-Phthalimido-1-acetoxy-cyclopentaneC15H15NO4ee >99%[α]D25=+10.0 (c 0.5, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1S,2S)
(1R,2R)-(−)-2-Phthalimidocyclohexan-1-olC14H15NO3ee >99%[α]D25=-34.0 (c 1, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1R,2R)
(1S,2S)-(+)-2-Phthalimido-1-acetoxy-cyclohexaneC16H17NO4ee >99%[α]D25=+7.5 (c 1, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1S,2S)
(1R,2R)-(−)-1-Phthalimidoindan-2-olC17H13NO3ee >99%[α]D25=-84.8 (c 0.25, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1R,2R)
(1S,2S)-(+)-1-Phthalimido-2-acetoxyindanC19H15NO4ee >99%[α]D25=+96.0 (c 0.1, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1S,2S)
(1R,2R)-(−)-2-Phthalimidoindan-1-olC17H13NO3ee 98%[α]D25=-23.0 (c 1, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1R,2R)
(1S,2S)-(+)-2-Phthalimido-1-acetoxyindanC19H15NO4ee 90%[α]D25=+20.0 (c 1, CHCl3)Source of chirality: enzymatic resolutionAbsolute configuration: (1S,2S)
Journal: Tetrahedron: Asymmetry - Volume 22, Issue 24, 31 December 2011, Pages 2134–2143