| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348237 | 980346 | 2006 | 6 صفحه PDF | دانلود رایگان |
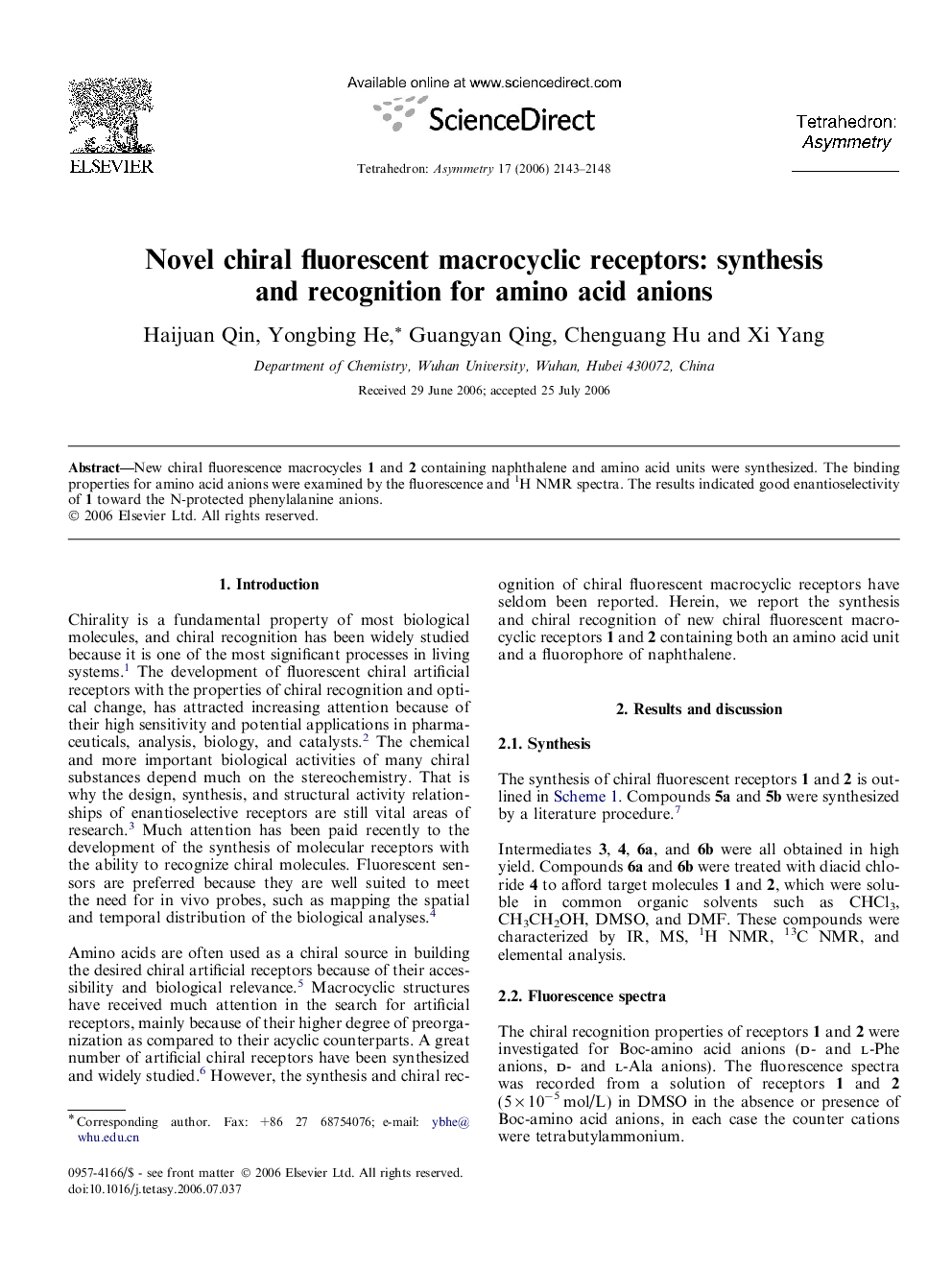
New chiral fluorescence macrocycles 1 and 2 containing naphthalene and amino acid units were synthesized. The binding properties for amino acid anions were examined by the fluorescence and 1H NMR spectra. The results indicated good enantioselectivity of 1 toward the N-protected phenylalanine anions.
Figure optionsDownload as PowerPoint slide
(6R,16R)-6,16-Methoxycarbonyl-2,11,20-trioxa-8,14-dithia-5,17-diaza-tricyclo[19.5.3.024,28]nonacosa-1(26),21(29),22,24,27-pentaene-4,18-dioneC26H32N2O9S2[α]D20=+43.0 (c 0.05, CHCl3)Source of chirality: l-cysteineAbsolute configuration: (R,R)
(6R,19R)-6,19-Methoxycarbonyl-2,11,14,23-tetraoxa-8,17-dithia-5,20-diaza-tricyclo[22.5.3.027,31]dotriaconta-1(29),24(32),25,27,30-pentaene-4,21-dioneC28H36N2O10S2[α]D20=+57.4 (c 0.05, CHCl3)Source of chirality: l-cysteineAbsolute configuration: (R,R)
Journal: Tetrahedron: Asymmetry - Volume 17, Issue 14, 28 August 2006, Pages 2143–2148