| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348324 | 980350 | 2007 | 4 صفحه PDF | دانلود رایگان |
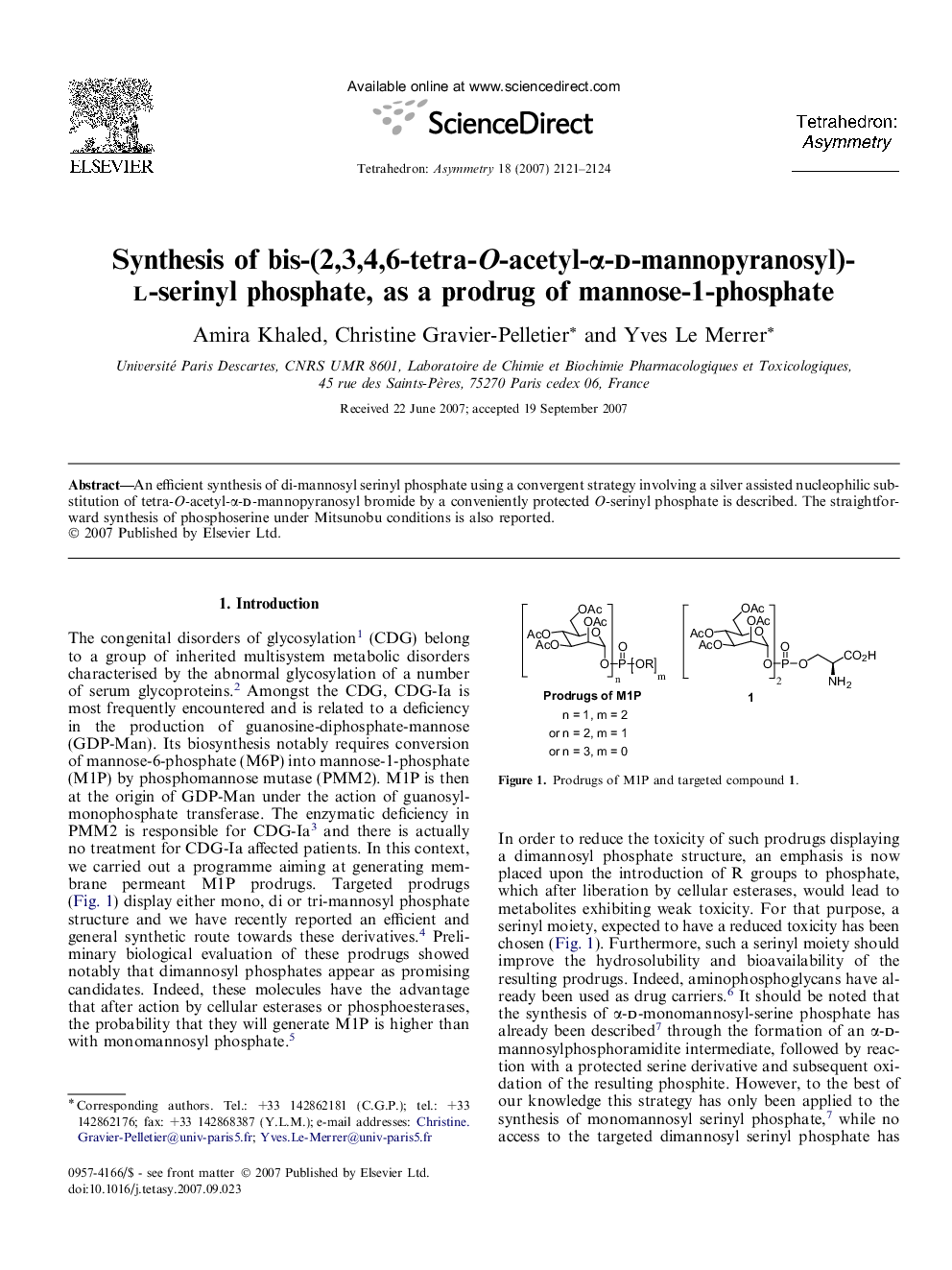
An efficient synthesis of di-mannosyl serinyl phosphate using a convergent strategy involving a silver assisted nucleophilic substitution of tetra-O-acetyl-α-d-mannopyranosyl bromide by a conveniently protected O-serinyl phosphate is described. The straightforward synthesis of phosphoserine under Mitsunobu conditions is also reported.
Figure optionsDownload as PowerPoint slide
2(S)-2-Benzyloxycarbonylamino-3-(di-tert-butoxy-phosphoryloxy)-propionic acid benzyl esterC26H36NO8PDe >95% (by 1H NMR)[α]D20=-6 (c 1.0, CH2Cl2)Source of chirality: N-benzyloxycarbonyl l-serine benzyl ester
2(S)-2-Benzyloxycarbonylamino-3-[bis-(2,3,4,6-tetra-O-acetyl-α-d-mannopyranosyloxy)phosphoryloxy]-propionic acid benzyl esterC46H56NO26PDe >95% (by 1H NMR)[α]D20=+45 (c 1.0, CH2Cl2)Source of chirality: N-benzyloxycarbonyl l-serine benzyl ester and 2,3,4,6-tetra-O-acetyl-α-d-manopyranosyl bromide
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 18, 17 September 2007, Pages 2121–2124