| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348328 | 980350 | 2007 | 8 صفحه PDF | دانلود رایگان |
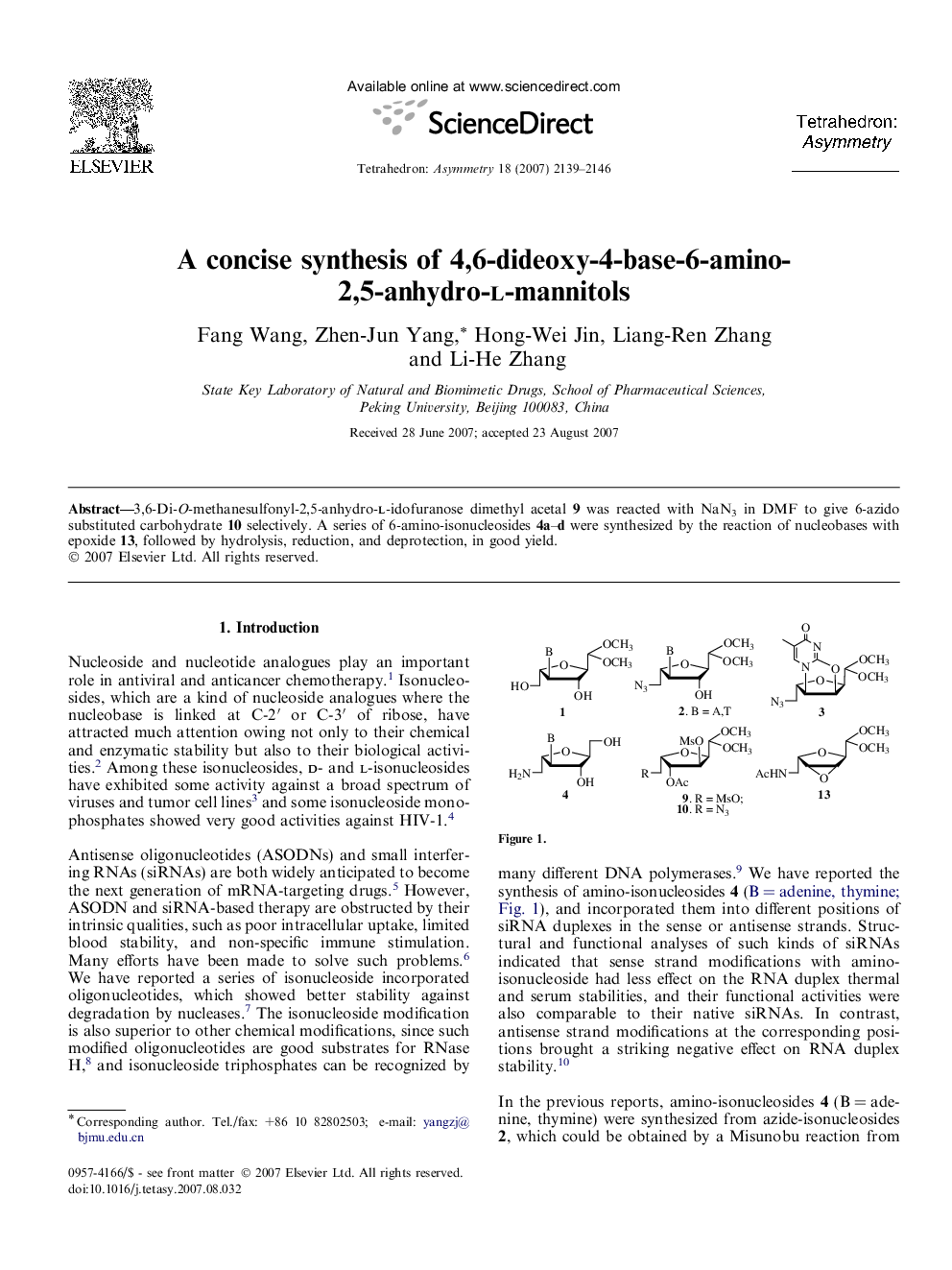
3,6-Di-O-methanesulfonyl-2,5-anhydro-l-idofuranose dimethyl acetal 9 was reacted with NaN3 in DMF to give 6-azido substituted carbohydrate 10 selectively. A series of 6-amino-isonucleosides 4a–d were synthesized by the reaction of nucleobases with epoxide 13, followed by hydrolysis, reduction, and deprotection, in good yield.
Figure optionsDownload as PowerPoint slide
3,6-Di-O-methanesulfonyl-2,5-anhydro-l-idofuranose dimethylacetalC10H20O10S2[α]D25=+10.1 (c 0.16, MeOH)Source of chirality: l-idofuranose
3-O-Methanesulfonyl-4-O-acetyl-6-deoxy-6-acetylamino-2,5-anhydro-l-idofuranose dimethylacetalC13H23NO9S[α]D25=-5.5 (c 0.42, MeOH)Source of chirality: l-idofuranose
6-Deoxy-6-acetylamino-2,5:3,4-dianhydro-l-talose dimethylacetalC10H17NO5[α]D25=+34.6 (c 0.47, MeOH)Source of chirality: l-talose
4,6-Dideoxy-4-(adenin-9-yl)-6-amino-2,5-anhydro-l-mannitolC11H16N6O3[α]D25=-11.0 (c 0.07, MeOH)Source of chirality: l-mannitol
4,6-Dideoxy-4-(cytosin-1-yl)-6-amino-2,5-anhydro-l-mannitolC10H16N4O4[α]D25=-6.4 (c 0.20, MeOH)Source of chirality: l-mannitol
4,6-Dideoxy-4-(5-fluorocytosin-1-yl)-6-amino-2,5-anhydro-l-mannitolC10H15FN4O4[α]D25=-15.0 (c 0.08, MeOH)Source of chirality: l-mannitol
4,6-Dideoxy-4-(thymin-1-yl)-6-amino-2,5-anhydro-l-mannitolC11H17N3O5[α]D25=-10.0 (c 0.07, MeOH)Source of chirality: l-mannitol
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 18, 17 September 2007, Pages 2139–2146