| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348337 | 980350 | 2007 | 7 صفحه PDF | دانلود رایگان |
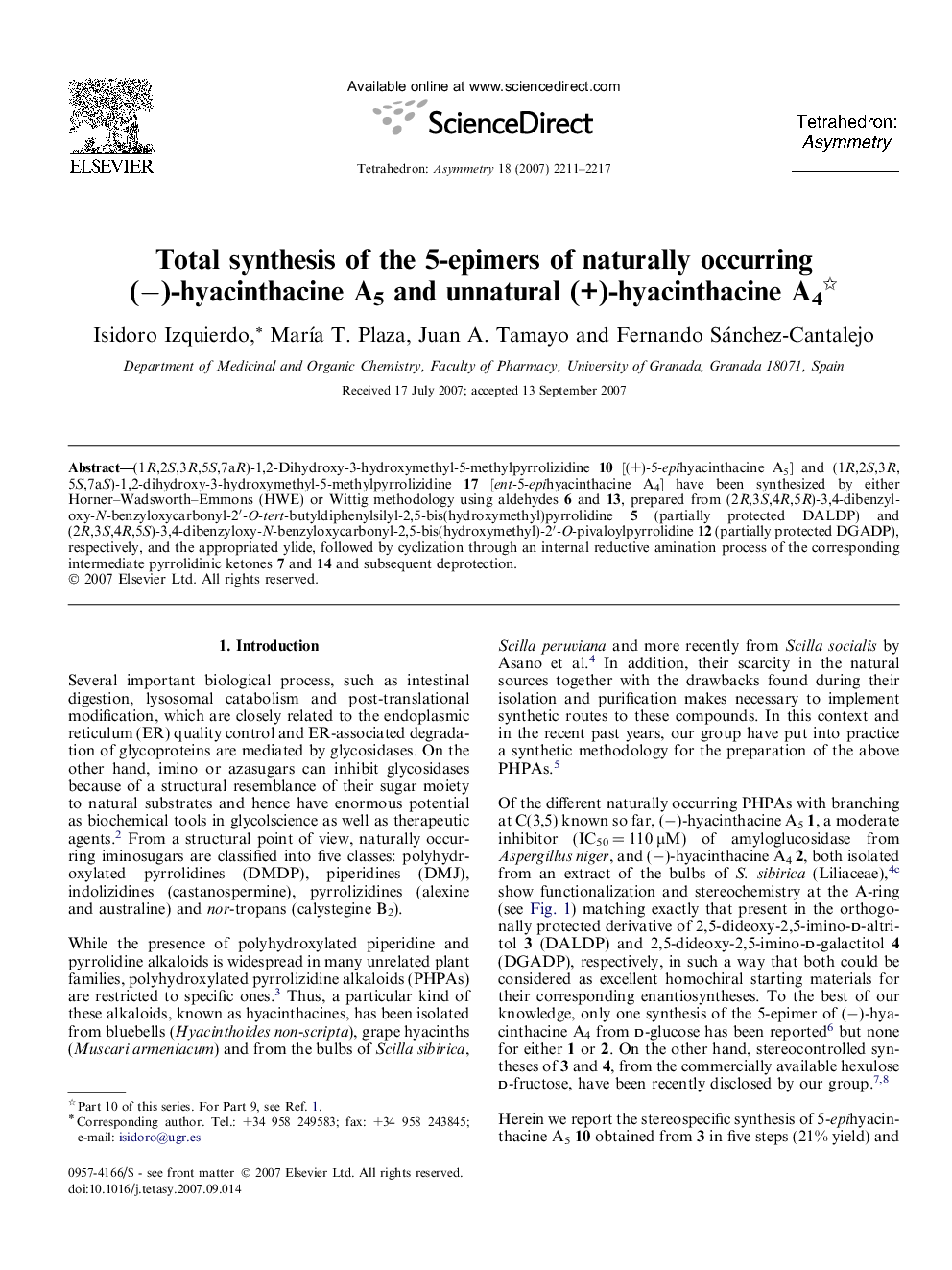
(1R,2S,3R,5S,7aR)-1,2-Dihydroxy-3-hydroxymethyl-5-methylpyrrolizidine 10 [(+)-5-epihyacinthacine A5] and (1R,2S,3R,5S,7aS)-1,2-dihydroxy-3-hydroxymethyl-5-methylpyrrolizidine 17 [ent-5-epihyacinthacine A4] have been synthesized by either Horner–Wadsworth–Emmons (HWE) or Wittig methodology using aldehydes 6 and 13, prepared from (2R,3S,4R,5R)-3,4-dibenzyloxy-N-benzyloxycarbonyl-2′-O-tert-butyldiphenylsilyl-2,5-bis(hydroxymethyl)pyrrolidine 5 (partially protected DALDP) and (2R,3S,4R,5S)-3,4-dibenzyloxy-N-benzyloxycarbonyl-2,5-bis(hydroxymethyl)-2′-O-pivaloylpyrrolidine 12 (partially protected DGADP), respectively, and the appropriated ylide, followed by cyclization through an internal reductive amination process of the corresponding intermediate pyrrolidinic ketones 7 and 14 and subsequent deprotection.
Figure optionsDownload as PowerPoint slide
(2R,3S,4R,5R)-3,4-Dibenzyloxy-N-benzyloxycarbonyl-2′-O-tert-butyldiphenylsilyl-2,5-bis(hydroxymethyl)pyrrolidineC44H49NO6Si[α]D25=+6, [α]40526=+21 (c 1, chloroform)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (2R,3S,4R,5R) (assigned by NMR spectroscopy and chemical correlation)
4-[(3E,2′R,3′R,4′S,5′R)-3′,4′-Dibenzyloxy-N-benzyloxycarbonyl-5′-tert-butyldiphenylsilyloxymethylpyrrolidin-2′-yl]but-3-en-2-oneC47H51NO6Si[α]D26=+18 (c 1, chloroform)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (3E,2′R,3′R,4′S,5′R) (assigned by NMR spectroscopy and chemical correlation)
(1R,2S,3R,5S,7aR)-1,2-Dibenzyloxy-3-tert-butyldiphenylsilyloxymethyl-5-methylpyrrolizidineC39H47NO3Si[α]D25=+31 (c 1, chloroform)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (1R,2S,3R,5S,7aR) (assigned by NMR spectroscopy and chemical correlation)
(1R,2S,3R,5S,7aR)-1,2-Dihydroxy-3-hydroxymethyl-5-methylpyrrolizidineC9H17NO3[α]D28=+12, [α]40528=-30 (c 1, methanol)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (1R,2S,3R,5S,7aR) (assigned by NMR spectroscopy and chemical correlation)
(2R,3S,4R,5S)-3,4-Dibenzyloxy-N-benzyloxycarbonyl-2,5-bis(hydroxymethyl)-2′-O-pivaloylpyrrolidineC33H39NO7[α]D23=+22 (c 1, chloroform)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (2R,3S,4R,5S) (assigned by NMR spectroscopy and chemical correlation)
4-[(3E,2′S,3′R,4′S,5′R)-3′,4′-Dibenzyloxy-N-benzyloxycarbonyl-5′-O-pivaloyloxymethylpyrrolidin-2′-yl]but-3-en-2-oneC36H41NO7[α]D26=-1, [α]40526=-116 (c 1, chloroform)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (3E,2′S,3′R,4′S,5′R) (assigned by NMR spectroscopy and chemical correlation)
(1R,2S,3R,5S,7aS)-1,2-Dibenzyloxy-5-methyl-3-pivaloyloxymethylpyrrolizidineC28H37NO4[α]D25=-27 (c 1, chloroform)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (1R,2S,3R,5S,7aS) (assigned by NMR spectroscopy and chemical correlation)
(1R,2S,3R,5S,7aS)-1,2-Dibenzyloxy-3-hydroxymethyl-5-methylpyrrolizidineC23H29NO3[α]D26=-26 (c 1, chloroform)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (1R,2S,3R,5S,7aS) (assigned by NMR spectroscopy and chemical correlation)
(1R,2S,3R,5S,7aS)-1,2-Dihydroxy-3-hydroxymethyl-5-methylpyrrolizidineC9H17NO3[α]D27=+10 (c 1, methanol)Source of chirality: d-fructose and stereoselective synthesisAbsolute configuration: (1R,2S,3R,5S,7aS) (assigned by NMR spectroscopy and chemical correlation)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 18, 17 September 2007, Pages 2211–2217