| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348697 | 980366 | 2007 | 7 صفحه PDF | دانلود رایگان |
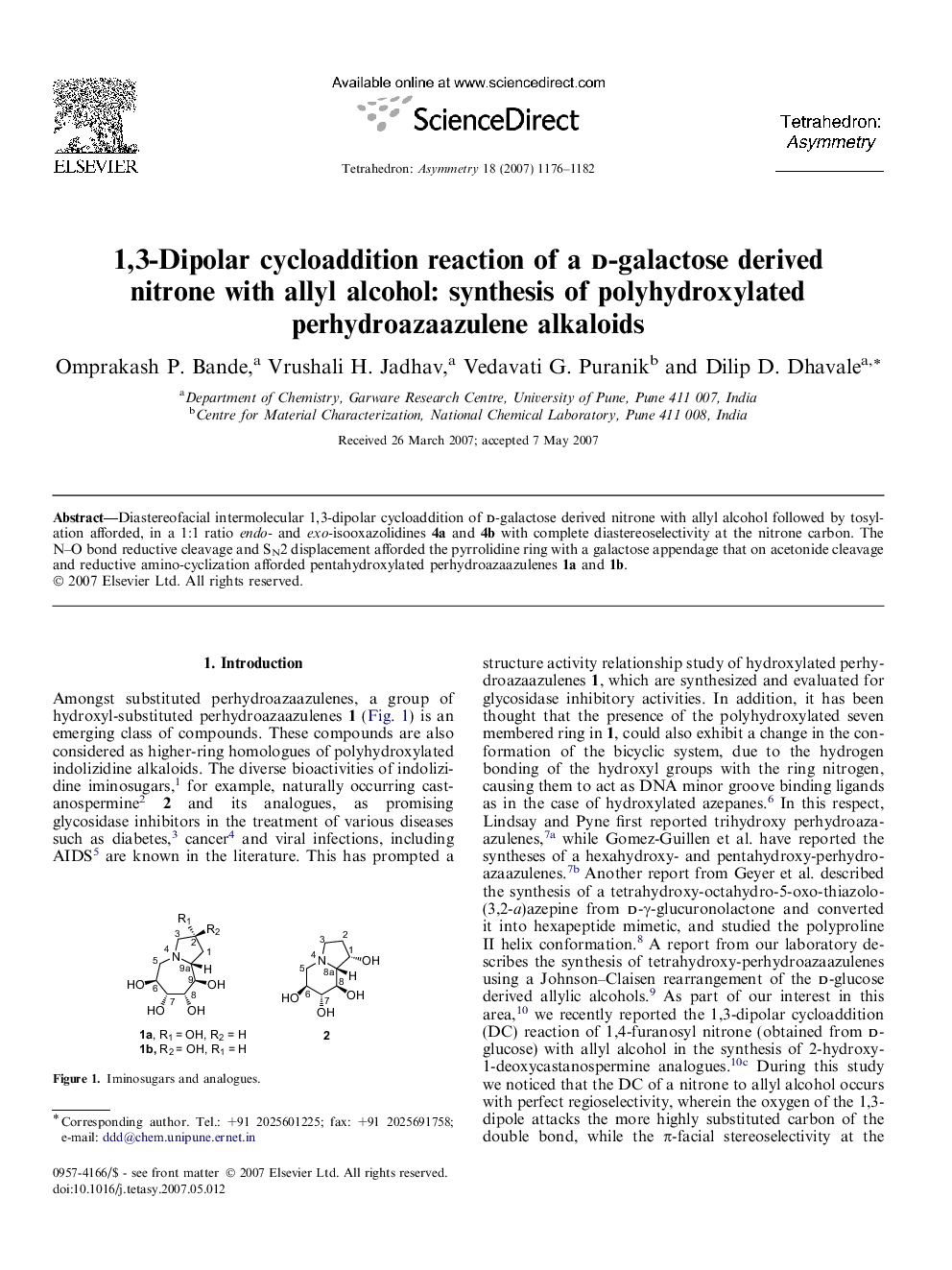
Diastereofacial intermolecular 1,3-dipolar cycloaddition of d-galactose derived nitrone with allyl alcohol followed by tosylation afforded, in a 1:1 ratio endo- and exo-isooxazolidines 4a and 4b with complete diastereoselectivity at the nitrone carbon. The N–O bond reductive cleavage and SN2 displacement afforded the pyrrolidine ring with a galactose appendage that on acetonide cleavage and reductive amino-cyclization afforded pentahydroxylated perhydroazaazulenes 1a and 1b.
Figure optionsDownload as PowerPoint slide
6,7,9-Trideoxy-6,9-(N-benzylimino)-1,2:3,4-di-O-isopropylidene-8(R)-hydroxy-β-l-threo-d-galacto-non-1,5-pyranoseC22H31NO6Ee = 100%[α]D25=-24 (c 0.41, CHCl3)Source of chirality:1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration:(1R,2R,3S,4S,5R,6R,8R)
6,7,9-Trideoxy-6,9-(N-benzylimino)-1,2:3,4-di-O-isopropylidene-8(S)-hydroxy-α-d-erythreo-d-galacto-non-1,5-pyranoseC22H31NO6Ee = 100%[α]D25=+36.0 (c 0.05, CHCl3)Source of chirality:1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration:(1R,2R,3S,4S,5R,6R,8S)
6,7,9-Trideoxy-6,9-(N-benzylimino)-1,2:3,4-di-O-isopropylidene-8(S)-hydroxy-α-d-erythreo-d-galacto-non-1,5-pyranoseC23H31NO8Ee = 100%[α]D25=+8.0 (c 0.05, CHCl3)Source of chirality:1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration:(1R,2R,3S,4S,5R,6R,8R)
6,7,9-Trideoxy-6,9-(N-benzylimino)-1,2:3,4-di-O-isopropylidene-8(R)-hydroxy-β-l-threo-d-galacto-non-1,5-pyranoseC22H31NO6Ee = 100%[α]D25=+400 (c 0.03, CHCl3)Source of chirality:1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration:(1R,2R,3S,4S,5R,6R,8R)
6,7,9-Trideoxy-6,9-(N-benzylimino)-1,2:3,4-di-O-isopropylidene-α-d-galacto-non-1,5-pyranoseC22H29NO6Ee = 100%[α]D25=+36 (c 0.05, CHCl3)Source of chirality:1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration:(1R,2R,3S,4S,5R,6R)
(2R,6S,7R,8S,9R,9aR)-2,6,7,8,9-Pentahydroxy-perhydroazaazuleneC9H17NO5Ee = 100%[α]D25=+7.0 (c 0.5, MeOH)Source of chirality: 1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration: (2R,6S,7R,8S,9R,9aR)
(2S,6S,7R,8S,9R,9aR)-2,6,7,8,9-Pentahydroxy-perhydroazaazuleneC9H17NO5Ee = 100%[α]D25=+90.9 (c 0.11, MeOH)Source of chirality: 1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration: (2S,6S,7R,8S,9R,9aR)
(2R,6S,7R,8S,9R,9aR)-2,6,7,8,9-Pentahydroxy-perhydroazaazuleneC19H27NO10Ee = 100%[α]D25=+208 (c 1.05, CHCl3)Source of chirality: 1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration: (2S,6S,7R,8S,9R,9aR)
(2S,6S,7R,8S,9R,9aR)-2,6,7,8,9-Penta-acetoxy-perhydroazaazuleneC19H27NO10Ee = 100%[α]D25=+324.3 (c 1.55, MeOH)Source of chirality: 1,2:3,4-di-O-isopropylidene-α-d-galactopyranoseAbsolute configuration: (2S,6S,7R,8S,9R,9aR)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 10, 11 June 2007, Pages 1176–1182