| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1348752 | 980369 | 2006 | 6 صفحه PDF | دانلود رایگان |
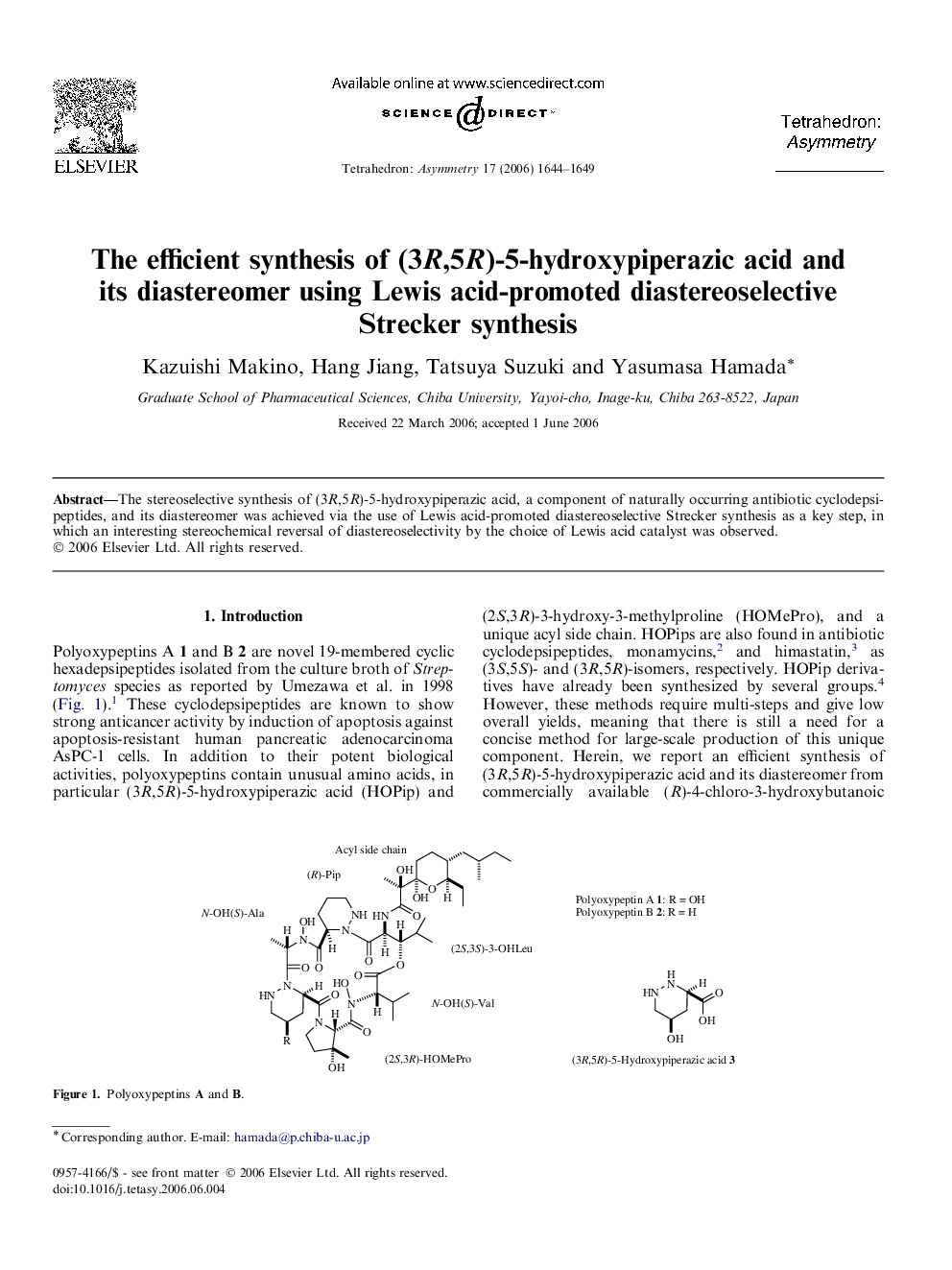
The stereoselective synthesis of (3R,5R)-5-hydroxypiperazic acid, a component of naturally occurring antibiotic cyclodepsipeptides, and its diastereomer was achieved via the use of Lewis acid-promoted diastereoselective Strecker synthesis as a key step, in which an interesting stereochemical reversal of diastereoselectivity by the choice of Lewis acid catalyst was observed.
Figure optionsDownload as PowerPoint slide
(R)-1-Benzoyl-5-(tert-butyldimethylsiloxy)-1,4,5,6-tetrahydropyridazine[α]D25=-26.7 (c 1.43, CHCl3)Source of chirality: ethyl (R)-4-chloro-3-hydroxybutyrate
(3R,5R)-1-Benzoyl-5-(tert-butyldimethylsiloxy)-hexahydropyridazine-3-carbonitrile[α]D25=+15.8 (c 0.85, CHCl3)Source of chirality: ethyl (R)-4-chloro-3-hydroxybutyrate
(3S,5R)-1-Benzoyl-5-(tert-butyldimethylsiloxy)-hexahydropyridazine-3-carbonitrile[α]D24=-26.7 (c 1.19, CHCl3)Source of chirality: ethyl (R)-4-chloro-3-hydroxybutyrate
(3R,5R)-1-tert-Butoxycarbonyl-5-hydroxypiperazic acid methyl ester[α]D25=+7.0 (c 1.00, CHCl3)Source of chirality: ethyl (R)-4-chloro-3-hydroxybutyrate
(3R,5S)-1-tert-Butoxycarbonyl-5-hydroxypiperazic acid methyl ester[α]D26=+1.96 (c 1.00, CHCl3)Source of chirality: ethyl (R)-4-chloro-3-hydroxybutyrate
(S)-1-Benzoyl-5-(tert-butyldimethylsiloxy)-1,4,5,6-tetrahydropyridazine[α]D25=+26.7 (c 1.43, CHCl3)Source of chirality: (S)-malic acid
(3S,5S)-1-Benzoyl-5-(tert-butyldimethylsiloxy)-hexahydropyridazine-3-carbonitrile[α]D25=-13.0 (c 0.89, CHCl3)Source of chirality: (S)-malic acid
(3R,5S)-1-Benzoyl-5-(tert-butyldimethylsiloxy)- hexahydropyridazine-3-carbonitrile[α]D24=+26.1 (c 2.10, CHCl3)Source of chirality: (S)-malic acid
Journal: Tetrahedron: Asymmetry - Volume 17, Issue 11, 17 July 2006, Pages 1644–1649