| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349039 | 980380 | 2010 | 8 صفحه PDF | دانلود رایگان |
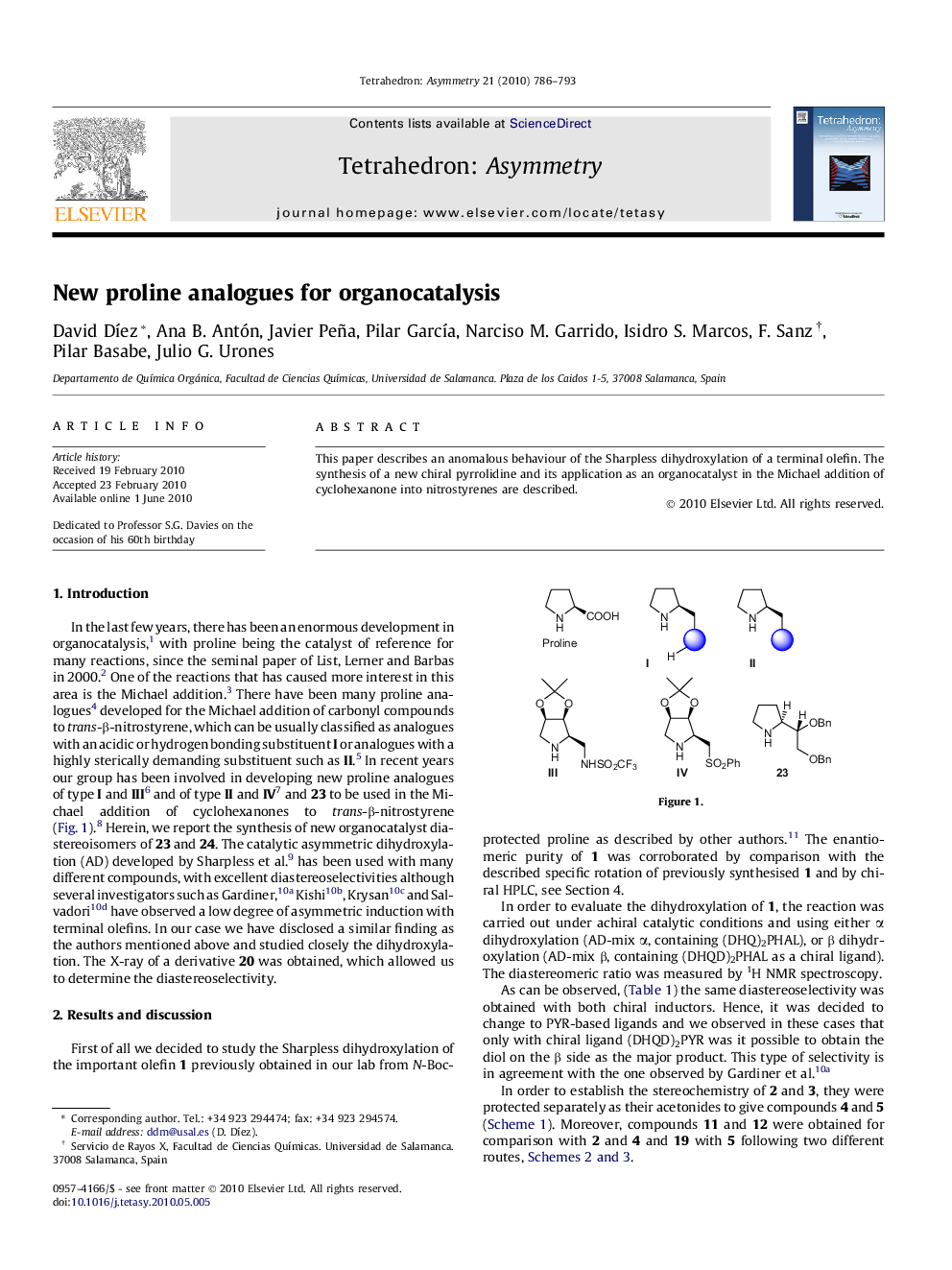
This paper describes an anomalous behaviour of the Sharpless dihydroxylation of a terminal olefin. The synthesis of a new chiral pyrrolidine and its application as an organocatalyst in the Michael addition of cyclohexanone into nitrostyrenes are described.
Figure optionsDownload as PowerPoint slide
(2S)-N-tert-Butoxycarbonyl-2-vinylpyrrolidineC11H19NO2Ee >95% (rotation, HPLC)[α]D20=-13.0 (c 0.91, CHCl3)Source of chirality: natural productAbsolute configuration: (2S)
(2S)-N-tert-Butoxycarbonyl-2-[(1′S)-1′,2′-dihydroxyethyl]-pyrrolidineC11H21NO4Ee, de >95% (NMR)[α]D20=-42.7 (c 1.1, CHCl3)Source of chirality: natural product, AD-mix αAbsolute configuration: (2S,1′S)
(2S)-N-tert-Butoxycarbonyl-2-[(1′R)-1′,2′-dihydroxyethyl]-pyrrolidineC11H21NO4Ee, de >95% (NMR)[α]D20=-74.1 (c 0.63, CHCl3)Source of chirality: natural product, Sharpless dihydroxylation (DHQD)2PYRAbsolute configuration: (2S,1′R)
(2S)-N-tert-Butoxycarbonyl-2-[(1′S)-1′,2′(isopropylidenedioxy)ethyl]-pyrrolidineC14H25NO4Ee, de >95% (NMR)[α]D20=-60.2 (c 1.25, CHCl3)Source of chirality: natural product, AD-mix αAbsolute configuration: (2S,1′S)
(2S)-N-tert-Butoxycarbonyl-2-[(1′R)-1′,2′(isopropylidenedioxy)ethyl]-pyrrolidineC14H25NO4Ee, de >95% (NMR)[α]D20=-84.3 (c 1.14, CHCl3)Source of chirality: natural product, Sharpless dihydroxylation (DHQD)2PYRAbsolute configuration: (2S,1′R)
(2R,3R)-3-(N-Allyl-N-tert-butoxycarbonylamino)-4-penten-1,2-diolC13H23NO4Ee, de >95% (NMR)[α]D20=+25.9 (c 1.15, CHCl3)Source of chirality: Sharpless epoxidationAbsolute configuration: (2R,3R)
(2R)-N-tert-Butoxycarbonyl-2-[(1′R)-1′,2′-dihydroxyethyl]-pyrrolidineC11H21NO4Ee, de >95% (NMR)[α]D20=+26.0 (c 0.95, CHCl3)Source of chirality: Sharpless epoxidationAbsolute configuration: (2R,1′R)
(2R)-N-tert-Butoxycarbonyl-2-[(1′R)-1′,2′(isopropylidenedioxy)ethyl]-pyrrolidineC14H25NO4Ee, de >95% (NMR)[α]D20=+48.2 (c 0.95, CHCl3)Source of chirality: Sharpless epoxidationAbsolute configuration: (2R,1′R)
(2R,3R)-3- (N-Allyl-N-tert-butoxycarbonylamino)-4-penten-1,2-diol acetonideC16H27NO4Ee, de >95% (NMR)[α]D20=+12.5 (c 0.95, CHCl3)Source of chirality: Sharpless epoxidationAbsolute configuration: (2R,3R)
(4R,5S)-4-Benzylamino-5,6-isopropylidenedioxy-1-hexyl-tert-butyldimethylsilyletherC22H39NO3SiEe, de >95% (NMR)[α]D20=+0.6 (c 1.05, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (4R,5S)
(4R,5S)-4-Benzylamino-5,6-isopropylidenedioxy-1-hexanolC16H25NO3Ee, de >95% (NMR)[α]D20=-11.0 (c 0.93, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (4R,5S)
(2R)-N-Benzyl-2-[(1′S)-1′,2′(isopropylidenedioxy)ethyl]-pyrrolidineC16H23NO2Ee, de >95% (NMR)[α]D20=+29.3 (c 0.96, CHCl3)Source of chirality: d-mannitolAbsolute configuration: (2R,1′S)
(4S,4aS)-4-Hydroxy-perhydropyrrolo[1,2-c]-1,3-oxazin-1-oneC7H11NO3Ee, de >95% (NMR)[α]D20=-33.7 (c 0.87, CHCl3)Source of chirality: natural product, AD-mix αAbsolute configuration: (4S,4aS)
(2S)-N-tert-Butoxycarbonyl-2-[(1′S)-1′,2′-dibenzyloxyethyl]-pyrrolidineC25H33NO4Ee, de >95% (NMR)[α]D20=-70.1 (c 0.72, CHCl3)Source of chirality: natural product, AD-mix αAbsolute configuration: (2S,1′S)
(2S)-N-tert-Butoxycarbonyl-2-[(1′R)-1′,2′-dibenzyloxyethyl]-pyrrolidineC25H33NO4Ee, de >95% (NMR)[α]D20=-31.7 (c 0.93, CHCl3)Source of chirality: natural product, Sharpless dihydroxylation (DHQD)2PYRAbsolute configuration: (2S,1′R)
(2S)-2-[(1′S)-1′,2′-Dibenzyloxyethyl]-pyrrolidineC20H25NO2Ee, de >95% (NMR)[α]D20=-34.6 (c 0.60, CHCl3)Source of chirality: natural product, AD-mix αAbsolute configuration: (2S,1′S)
(2S)-2-[(1′R)-1′, 2′-Dibenzyloxyethyl]-pyrrolidineC20H26NO2Ee, de >95% (NMR)[α]D20=+7.3 (c 0.9, CHCl3)Source of chirality: natural product, Sharpless dihydroxylation (DHQD)2PYRAbsolute configuration: (2S,1′R)
Journal: Tetrahedron: Asymmetry - Volume 21, Issue 7, 21 April 2010, Pages 786–793