| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349073 | 980381 | 2007 | 9 صفحه PDF | دانلود رایگان |
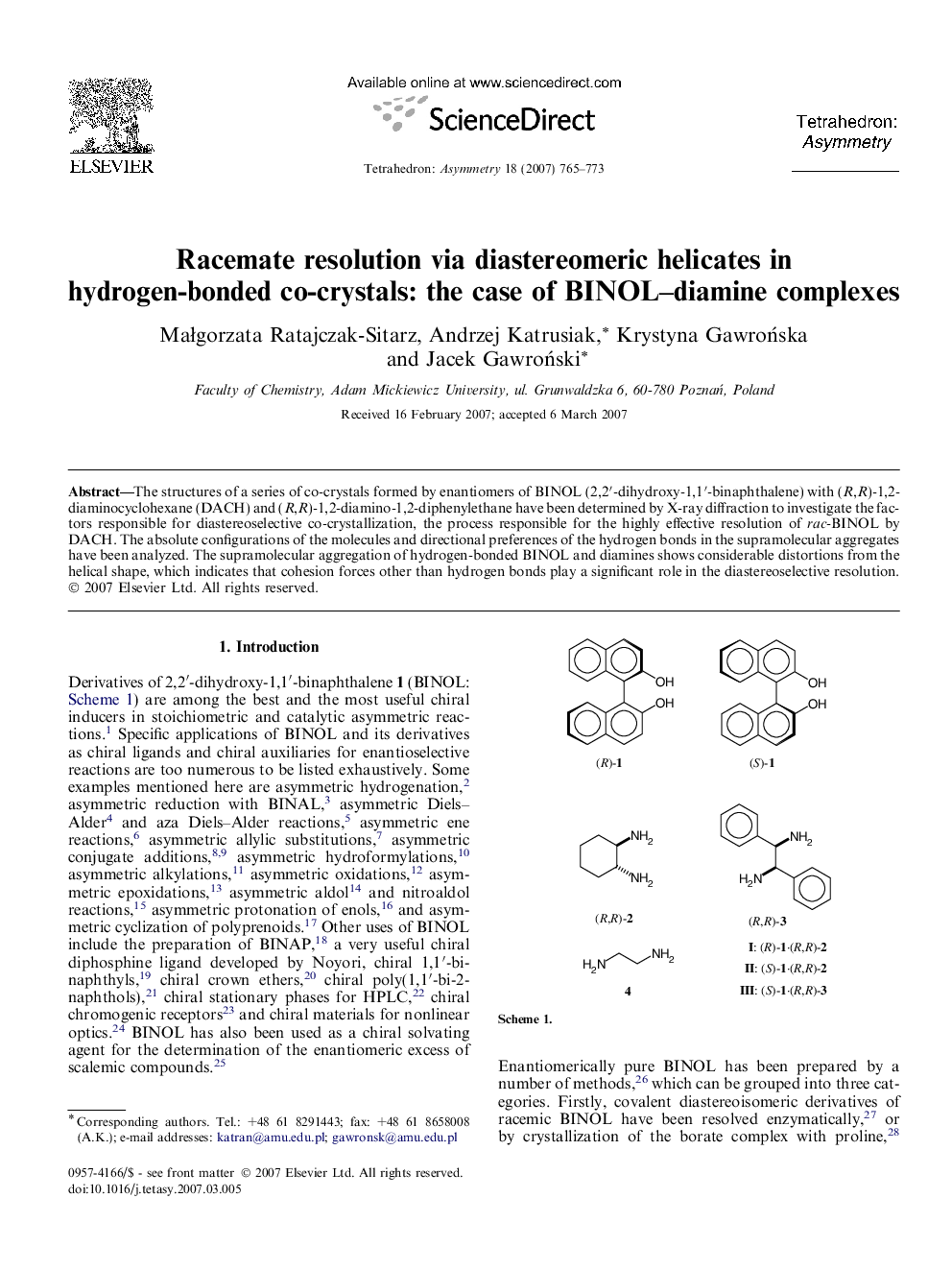
The structures of a series of co-crystals formed by enantiomers of BINOL (2,2′-dihydroxy-1,1′-binaphthalene) with (R,R)-1,2-diaminocyclohexane (DACH) and (R,R)-1,2-diamino-1,2-diphenylethane have been determined by X-ray diffraction to investigate the factors responsible for diastereoselective co-crystallization, the process responsible for the highly effective resolution of rac-BINOL by DACH. The absolute configurations of the molecules and directional preferences of the hydrogen bonds in the supramolecular aggregates have been analyzed. The supramolecular aggregation of hydrogen-bonded BINOL and diamines shows considerable distortions from the helical shape, which indicates that cohesion forces other than hydrogen bonds play a significant role in the diastereoselective resolution.
Figure optionsDownload as PowerPoint slide
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 6, 16 April 2007, Pages 765–773