| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349077 | 980381 | 2007 | 6 صفحه PDF | دانلود رایگان |
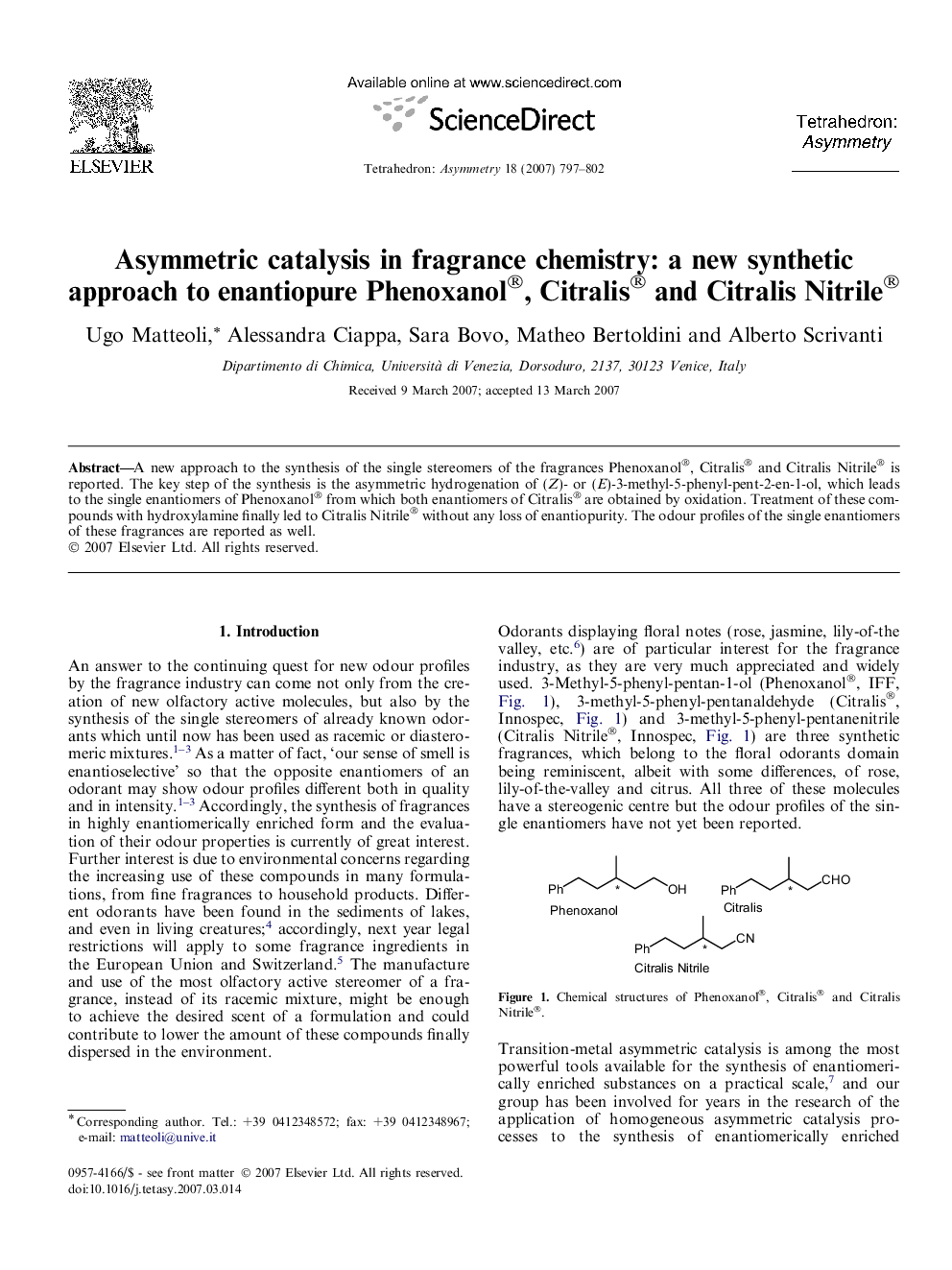
A new approach to the synthesis of the single stereomers of the fragrances Phenoxanol®, Citralis® and Citralis Nitrile® is reported. The key step of the synthesis is the asymmetric hydrogenation of (Z)- or (E)-3-methyl-5-phenyl-pent-2-en-1-ol, which leads to the single enantiomers of Phenoxanol® from which both enantiomers of Citralis® are obtained by oxidation. Treatment of these compounds with hydroxylamine finally led to Citralis Nitrile® without any loss of enantiopurity. The odour profiles of the single enantiomers of these fragrances are reported as well.
Figure optionsDownload as PowerPoint slide
(R)-3-Methyl-5-phenylpentan-1-olC12H18OEe >97% ee[α]D25=+14.5 (c 5, dichloromethane)Source of chirality: asymmetric hydrogenationAbsolute configuration: (R)
(R)-3-Methyl-5-phenylpentanalC12H16OEe >97% ee[α]D25=+22.9 (c 1, dichloromethane)Source of chirality: asymmetric hydrogenationAbsolute configuration: (R)
(R)-3-Methyl-5-phenylpentanenitrileC12H15NEe >97% ee[α]D25=-2.3 (c 2.2, ethanol)Source of chirality: asymmetric hydrogenationAbsolute configuration: (R)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 6, 16 April 2007, Pages 797–802