| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349125 | 980384 | 2007 | 6 صفحه PDF | دانلود رایگان |
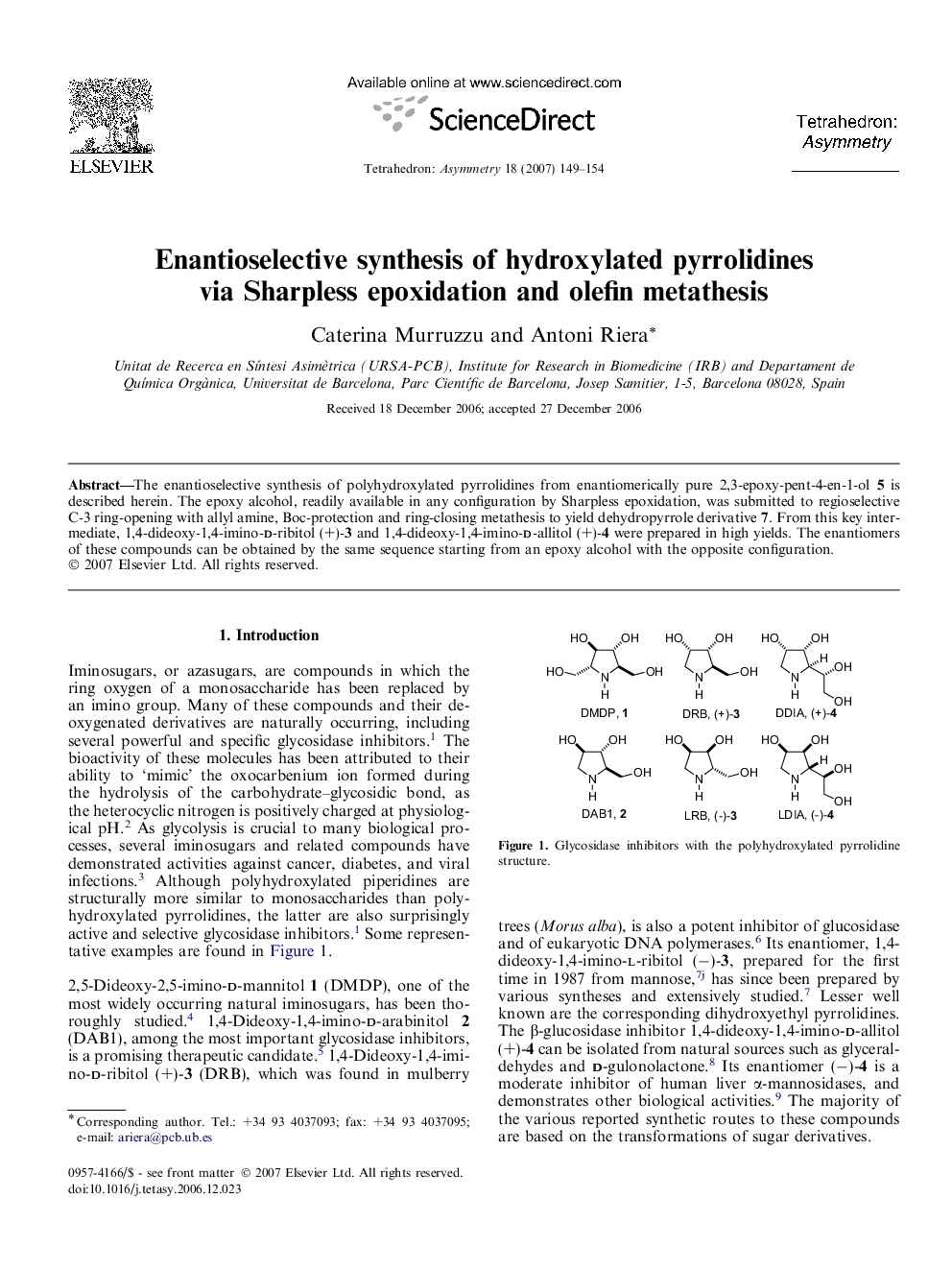
The enantioselective synthesis of polyhydroxylated pyrrolidines from enantiomerically pure 2,3-epoxy-pent-4-en-1-ol 5 is described herein. The epoxy alcohol, readily available in any configuration by Sharpless epoxidation, was submitted to regioselective C-3 ring-opening with allyl amine, Boc-protection and ring-closing metathesis to yield dehydropyrrole derivative 7. From this key intermediate, 1,4-dideoxy-1,4-imino-d-ribitol (+)-3 and 1,4-dideoxy-1,4-imino-d-allitol (+)-4 were prepared in high yields. The enantiomers of these compounds can be obtained by the same sequence starting from an epoxy alcohol with the opposite configuration.
Figure optionsDownload as PowerPoint slide
(2S,3S)-3-(N-Allyl-N-tert-butoxycarbonyl)-4-penten-1,2-diolC13H23NO4[α]D = −22.6 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S,3S)
(2S)-N-tert-Butoxycarbonyl-2-[(1′S)-1′,2′-dihydroxyethyl]-2,5-dihydropyrroleC11H19NO4[α]D = −108.1 (c 0.97, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S,1′S)
1,4-Dideoxy-1,4-imino-d-allitol bis-isopropylidene acetalC17H29NO6[α]D = −58.5 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R,3R,4S,1’S)
(2S)-N-tert-Butoxycarbonyl-2-hydroxymethyl-2,5-dihydro-1H-pyrroleC10H17NO3[α]D = −124.6 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2S)
(2R,3R,4S)-N-tert-Butoxycarbonyl-2-hydroxymethyl-3,4-isopropylidendioxy-pyrrolidineC13H23NO3[α]D = −30.3 (c 0.3, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R,3R,4S)
(2R)-N-tert-Butoxycarbonyl-2-tert-butyldiphenylsilyloxymethyl-2,5-dihydro-1H- pyrroleC26H36NO3Si[α]D = −24.6 (c 1.0, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R)
(2R,3R,4S)-N-tert-Butoxycarbonyl-2-tert-butyldiphenylsilyloxymethyl-3,4-dihydroxypyrrolidine isopropyilidene acetalC29H41NO5Si[α]D = −36.1 (c 1.05, CHCl3)Source of chirality: Sharpless asymmetric epoxidationAbsolute configuration: (2R,3R,4S)
Journal: Tetrahedron: Asymmetry - Volume 18, Issue 1, 31 January 2007, Pages 149–154