| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349248 | 980390 | 2010 | 5 صفحه PDF | دانلود رایگان |
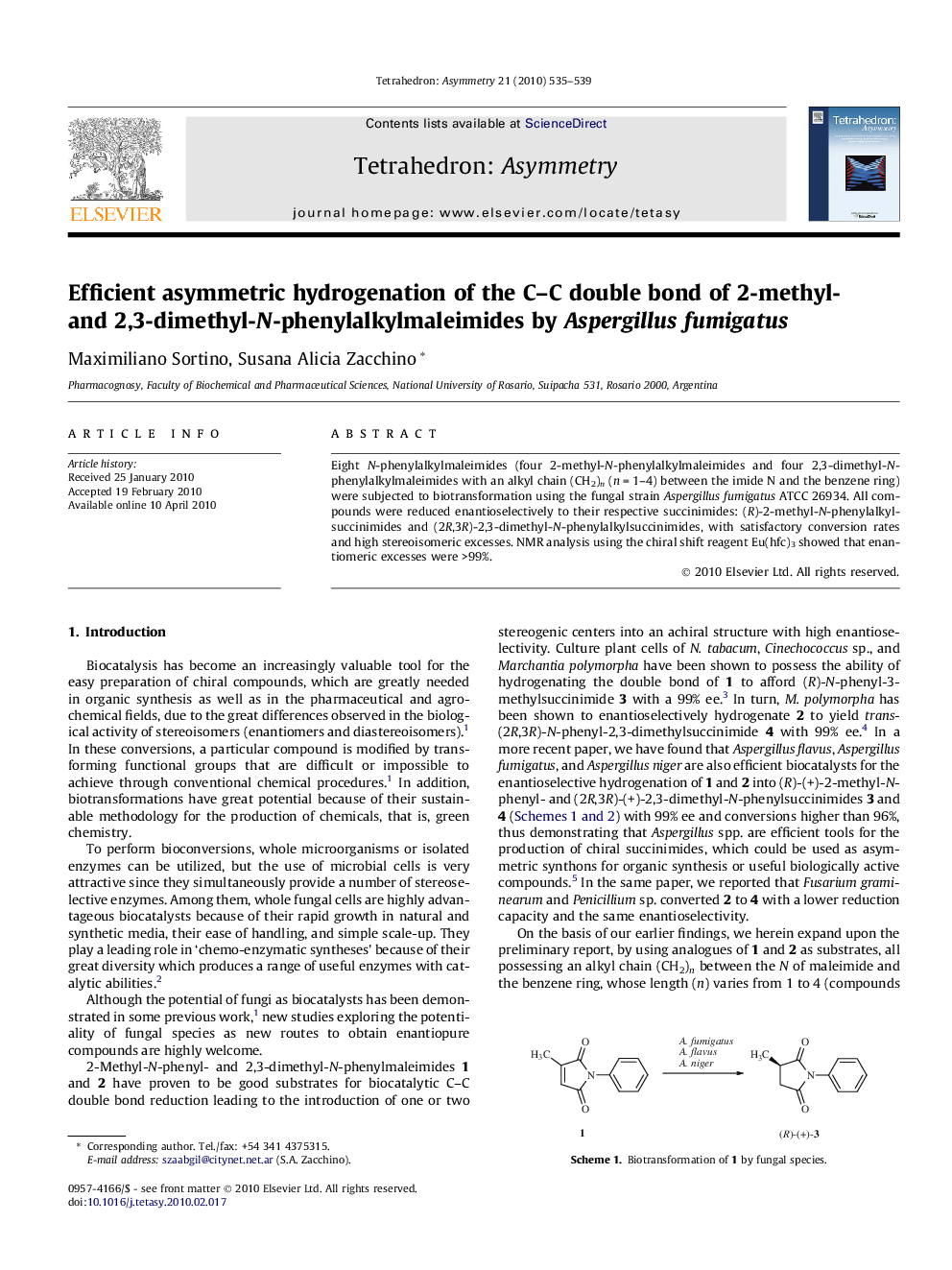
Eight N-phenylalkylmaleimides (four 2-methyl-N-phenylalkylmaleimides and four 2,3-dimethyl-N-phenylalkylmaleimides with an alkyl chain (CH2)n (n = 1–4) between the imide N and the benzene ring) were subjected to biotransformation using the fungal strain Aspergillus fumigatus ATCC 26934. All compounds were reduced enantioselectively to their respective succinimides: (R)-2-methyl-N-phenylalkylsuccinimides and (2R,3R)-2,3-dimethyl-N-phenylalkylsuccinimides, with satisfactory conversion rates and high stereoisomeric excesses. NMR analysis using the chiral shift reagent Eu(hfc)3 showed that enantiomeric excesses were >99%.
Figure optionsDownload as PowerPoint slide
(+)-2-Methyl-N-bencylsuccinimideC12H13NO2Ee >99%[α]D27=+19.7±1.2 (c 0.73, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (R)
(+)-2-Methyl-N-phenethylsuccinimideC13H15NO2Ee >99%[α]D27=+7.0±1.5 (c 0.73, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (R)
(+)-2-Methyl-N-propylphenylsuccinimideC14H17NO2Ee >99%[α]D27=+1.5±0.8 (c 1.50, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (R)
(+)-2-Methyl-N-butylphenylsuccinimideC15H19NO2Ee >99%[α]D27=+1.3±0.3 (c 0.45, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (R)
(+)-2,3-Dimethyl-N-bencylsuccinimideC13H15NO2Ee >99%[α]D27=+38.0±2.2 (c 0.31, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (2R,3R)
(+)-2,3-Dimethyl-N-phenethylsuccinimideC14H17NO2Ee >99%[α]D27=+40.8±0.6 (c 0.36, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (2R,3R)
(+)-2,3-Dimethyl-N-propylphenylsuccinimideC15H19NO2Ee >99%[α]D27=+39.4±0.90 (c 0.36, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (2R,3R)
(+)-2,3-Dimethyl-N-butylphenylsuccinimideC16H21NO2Ee >99%[α]D27=+38.3±0.6 (c 0.36, CHCl3)Source of chirality: hydrogenation by AspergillusfumigatusAbsolute configuration: (2R,3R)
Journal: Tetrahedron: Asymmetry - Volume 21, Issue 5, 30 March 2010, Pages 535–539