| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349496 | 980402 | 2006 | 4 صفحه PDF | دانلود رایگان |
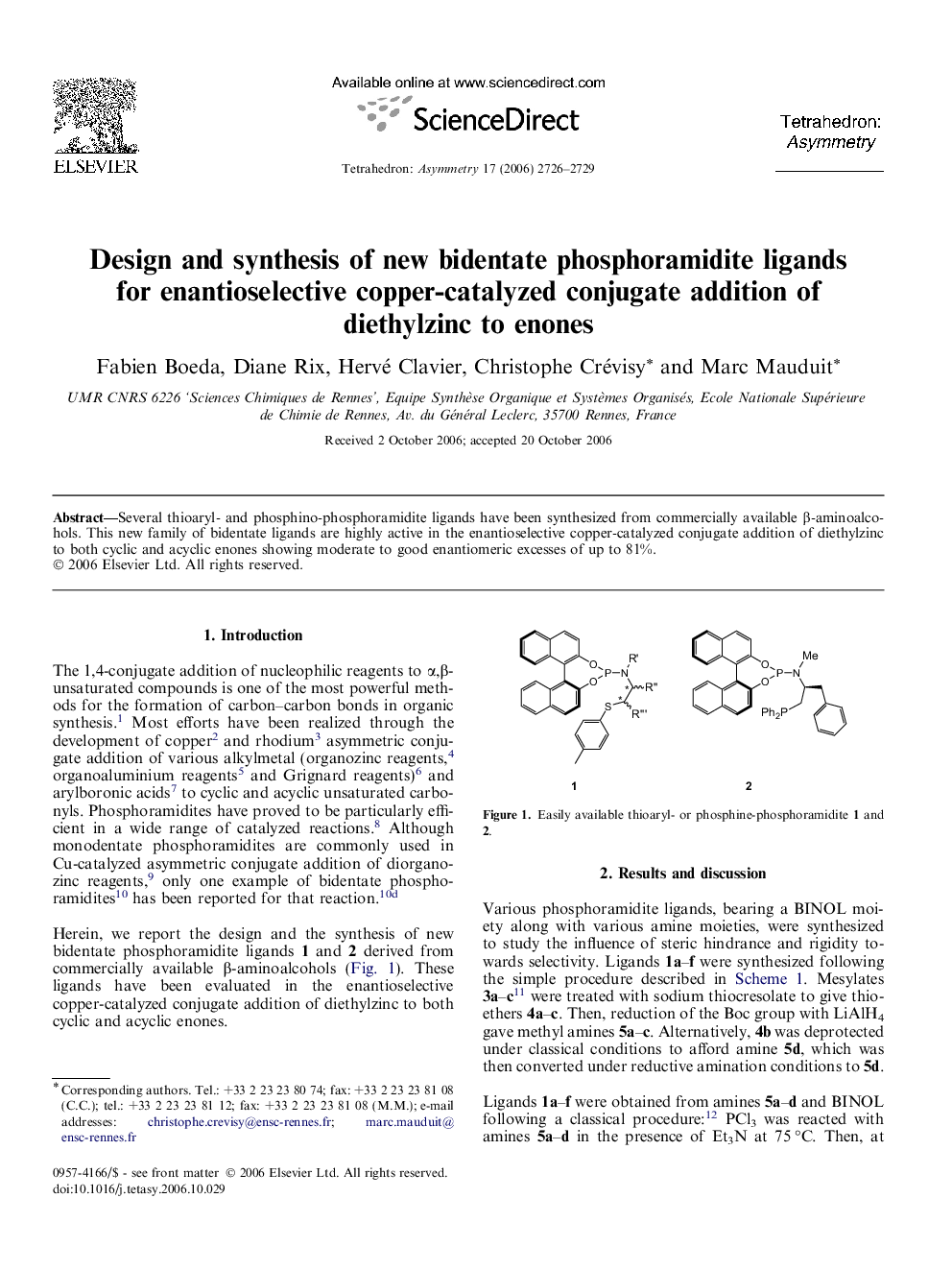
Several thioaryl- and phosphino-phosphoramidite ligands have been synthesized from commercially available β-aminoalcohols. This new family of bidentate ligands are highly active in the enantioselective copper-catalyzed conjugate addition of diethylzinc to both cyclic and acyclic enones showing moderate to good enantiomeric excesses of up to 81%.
Figure optionsDownload as PowerPoint slide
O,O′-(R)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-(S)-1-phenyl-3-(p-tolylthio)propanephosphoramiditeC37H32NO2PS[α]20 = −246.0 (c 1.7, chloroform)Source of chirality: (R)-BINOL 99% ee, (S)-phenylalaninol 99% eeAbsolute configuration: (R,S)
O,O′-(S)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-(S)-1-phenyl-3-(p-tolylthio)propanephosphoramiditeC37H32NO2PS[α]20 = +250.0 (c 1.2, chloroform)Source of chirality: (S)-BINOL 99% ee, (S)-phenylalaninol 99% eeAbsolute configuration: (S,S)
O,O′-(S)-(1,1′-Dinaphtyl-2,2′-diyl)-N-benzyl-N′-(S)-1-phenyl-3-(p-tolylthio)propanephosphoramiditeC43H36NO2PS[α]20 = +258.2 (c 1.7, chloroform)Source of chirality: (S)-BINOL 99% ee, (S)-phenylalaninol 99% eeAbsolute configuration: (S,S)
O,O′-(R)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-2-(p-tolylthio)ethanephosphoramiditeC30H26NO2PS[α]20 = −54.3 (c 0.53, chloroform)Source of chirality: (R)-BINOL 99% eeAbsolute configuration: (R)
O,O′-(S)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-(S)-1,1′,1″-trimethyl-3-(p-tolylthio)propanephosphoramiditeC34H34NO2PS[α]20 = +102.6 (c 0.70, chloroform)Source of chirality: (S)-BINOL 99% ee, (S)-tert-leucinol 99% eeAbsolute configuration: (S,S)
O,O′-(R)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-(S)-1,1′,1″-trimethyl-3-(p-tolylthio)propanephosphoramiditeC34H34NO2PS[α]20 = −99.2(c 0.60, chloroform)Source of chirality: (R)-BINOL 99% ee, (S)-tert-leucinol 99% eeAbsolute configuration: R,S
O,O′-(R)-(1,1′-Dinaphtyl-2,2′-diyl)-N-benzyl-N′-2-(methylthio)phenyl phosphoramiditeC34H26NO2PS[α]20 = −113.4 (c 0.50, chloroform)Source of chirality: (R)-BINOL 99% eeAbsolute configuration: R
O,O′-(R)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-(1R,2R)-2-(p-tolylthio)-2,3-dihydro-1H-inden-1-yl phosphoramiditeC37H30NO2PS[α]20 = −39.4 (c 0.66, chloroform)Source of chirality: (R)-BINOL 99% ee, (1R,2S)-cis-1-amino-2-indanol 99% eeAbsolute configuration: (R,R,R)
O,O′-(S)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-(S)-1-phenyl-3-(diphenylphosphino)propanephosphoramiditeC42H35NO2P2[α]20 = +58.5 (c 0.20, chloroform)Source of chirality: (S)-BINOL 99% ee, (S)-phenylalaninol 99% eeAbsolute configuration: (S,S)
O,O′-(R)-(1,1′-Dinaphtyl-2,2′-diyl)-N-methyl-N′-2-(diphenylphosphino)ethanephosphoramiditeC35H29NO2P2[α]20 = −70.7 (c 0.20, chloroform)Source of chirality: (R)-BINOL 99% eeAbsolute configuration: (R)
O,O′-(S)-(1,1′-Dinaphtyl-2,2′-diyl)-N-benzyl-N′-2-(o-diphenylphosphino)benzyl phosphoramiditeC46H35NO2P2[α]20 = +38.0 (c 0.33, chloroform)Source of chirality: (S)-BINOL 99% eeAbsolute configuration: (S)
Journal: Tetrahedron: Asymmetry - Volume 17, Issue 19, 27 October 2006, Pages 2726–2729