| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349844 | 1500371 | 2009 | 8 صفحه PDF | دانلود رایگان |
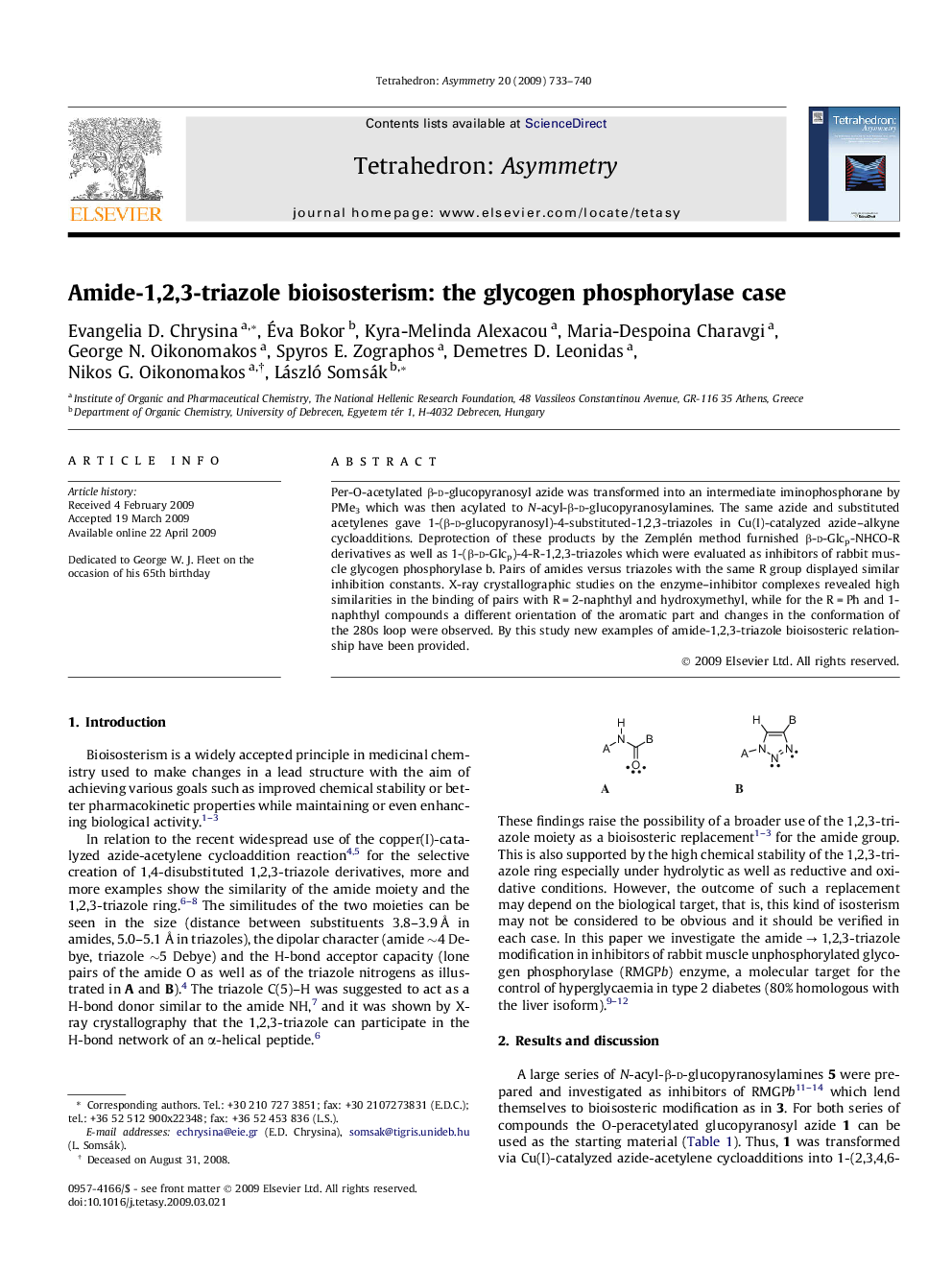
Per-O-acetylated β-d-glucopyranosyl azide was transformed into an intermediate iminophosphorane by PMe3 which was then acylated to N-acyl-β-d-glucopyranosylamines. The same azide and substituted acetylenes gave 1-(β-d-glucopyranosyl)-4-substituted-1,2,3-triazoles in Cu(I)-catalyzed azide–alkyne cycloadditions. Deprotection of these products by the Zemplén method furnished β-d-Glcp-NHCO-R derivatives as well as 1-(β-d-Glcp)-4-R-1,2,3-triazoles which were evaluated as inhibitors of rabbit muscle glycogen phosphorylase b. Pairs of amides versus triazoles with the same R group displayed similar inhibition constants. X-ray crystallographic studies on the enzyme–inhibitor complexes revealed high similarities in the binding of pairs with R = 2-naphthyl and hydroxymethyl, while for the R = Ph and 1-naphthyl compounds a different orientation of the aromatic part and changes in the conformation of the 280s loop were observed. By this study new examples of amide-1,2,3-triazole bioisosteric relationship have been provided.
Kinetic measurements and X-ray crystallographic studies of rabbit muscle glycogen phosphorylase b enzyme-inhibitor complexes prove bioisosteric relationship of N-acyl-β-d-glucopyranosylamines (A) and 1-(β-d-glucopyranosyl)-4-substituted-1,2,3-triazoles (B).Figure optionsDownload as PowerPoint slide
N-benzoyl-β-d-glucopyranosylamineC13H17NO6[α]D = +6 (c 1.0, MeOH)
N-(1-naphthoyl)-β-d-glucopyranosylamineC17H19NO6[α]D = +45 (c 0.16, DMSO)
N-(2-naphthoyl)-β-d-glucopyranosylamineC17H19NO6[α]D = +26 (c 0.2, DMSO)
N-hydroxyacetyl-β-d-glucopyranosylamineC8H15NO7[α]D = −2 (c 0.22, MeOH)
1-(β-d-glucopyranosyl)-4-phenyl-1,2,3-triazoleC14H17N3O5[α]D = −69 (c 0.24, DMSO)
1-(β-d-glucopyranosyl)-4-(1-naphthyl)-1,2,3-triazoleC18H19N3O5[α]D = −22 (c 0.21, MeOH)
1-(β-d-glucopyranosyl)-4-(2-naphthyl)-1,2,3-triazoleC18H19N3O5[α]D = −26 (c 0.21, DMSO)
1-(β-d-glucopyranosyl)-4-hydroxymethyl-1,2,3-triazoleC9H15N3O6[α]D = −5 (c 0.16, H2O)
Journal: Tetrahedron: Asymmetry - Volume 20, Issues 6–8, 7 May 2009, Pages 733–740