| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349854 | 1500371 | 2009 | 11 صفحه PDF | دانلود رایگان |
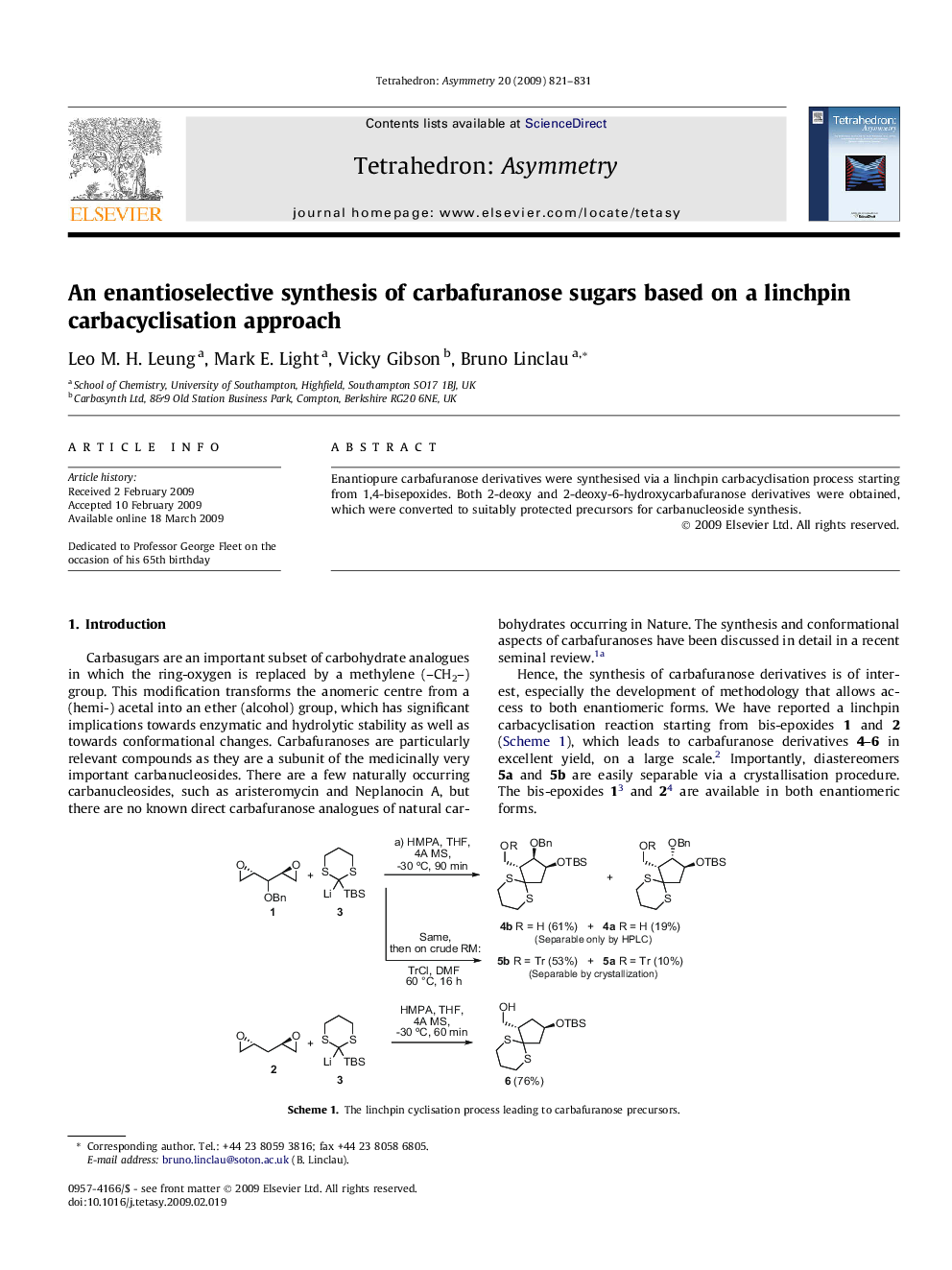
Enantiopure carbafuranose derivatives were synthesised via a linchpin carbacyclisation process starting from 1,4-bisepoxides. Both 2-deoxy and 2-deoxy-6-hydroxycarbafuranose derivatives were obtained, which were converted to suitably protected precursors for carbanucleoside synthesis.
Enantiopure carbafuranose derivatives were synthesised via a linchpin carbacyclisation process starting from 1,4-bisepoxides. Both 2-deoxy and 2-deoxy-6-hydroxycarbafuranose derivatives were obtained, which were converted to suitably protected precursors for carbanucleoside synthesis.Figure optionsDownload as PowerPoint slide
(2S,4R)-[4-(tert-Butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentan-1-one-1,3-propanedithioketalC34H44O2S2SiEe = 100%[α]D = −16.6 (c 0.67, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2S,4R)
(2S,4R)-[4-(tert-Butyldimethylsilyloxy)-2-benzyloxymethyl]cyclopentan-1-one-1,3-propanedithioketalC22H36O2S2SiEe = 100%[α]D = −10.8 (c 0.6, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2S,4R)
(2S,4R)-[4-(tert-Butyldimethylsilyloxy)-2-benzyloxymethyl]cyclopentanoneC19H30O3SiEe = 100%[α]D = −72.6 (c 0.9, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2S,4R)
(2R,3R,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanoneC38H44O4SiEe = 100%[α]D = −6.3 (c 1.1, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2R,3R,4S)
(2R,3S,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanoneC34H44O2S2SiEe = 100%[α]D = −23.7 (c 0.45, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2R,3S,4S)
(2S,4R)-[4-(tert-Butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanoneC31H38O3SiEe = 100%[α]D = −48.7 (c 1.1, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2S,4R)
(2R,3R,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-hydroxymethyl]cyclopentanoneC19H30O4SiEe = 100%[α]D = +3.8 (c 0.55, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2R,3R,4S)
(2R,3S,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-hydroxymethyl]cyclopentanoneC19H30O4SiEe = 100%[α]D = −88.3 (c 0.35, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2R,3S,4S)
(2R,4S)-[4-(tert-Butyldimethylsilyloxy)-2-hydroxymethyl]cyclopentanoneC12H24O3SiEe = 100%[α]D = +99.0 (c 0.26 CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (2R,4S)
(1R,2S,4R)-[4-(tert-Butyldimethylsilyloxy)-2-hydroxymethyl]cyclopentanolC12H26O3SiEe = 100%[α]D = −7.9 (c 0.98, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,4R)
(1R,2S,3R,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-hydroxymethyl]cyclopentanolC19H32O4SiEe = 100%[α]D = +82.0 (c 1.5, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3R,4S)
(1R,2S,3S,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-hydroxymethyl]cyclopentanolC19H32O4SiEe = 100%[α]D = −29.8 (c 1.2, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3S,4S)
(1S,2S,4R)-[4-(tert-Butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanolC31H40O3SiEe = 100%[α]D = +1.7 (c 1.1, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1S,2S,4R)
(1R,2S,4R)-[4-(tert-Butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanolC31H40O3SiEe = 100%[α]D = +30.5 (c 0.32, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,4R)
(1S,2S,3R,4S)-3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanolC38H46O4SiEe = 100%[α]D = +42.6 (c 0.95, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1S,2S,3R,4S)
(1R,2S,3R,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanolC38H46O4SiEe = 100%[α]D = +66.1 (c 0.80, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3R,4S)
(1S,2S,3S,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanolC38H46O4SiEe = 100%[α]D = +13.3 (c 0.45, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1S,2S,3S,4S)
(1R,2S,3S,4S)-[3-Benzyloxy-4-(tert-butyldimethylsilyloxy)-2-trityloxymethyl]cyclopentanolC38H46O4SiEe = 100%[α]D = +23.6 (c 0.35, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3S,4S)
(1S,2R,3S,4S)-[(2-Benzyloxy-4-(2-(methoxy)ethoxymethoxy)-3-trityloxymethyl)cyclopentyloxy]tert-butyldimethylsilaneC42H54O6SiEe = 100%[α]D = +23.7 (c 0.35, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1S,2R,3S,4S)
(1S,2S,3S,4S)-[(2-Benzyloxy-4-(2-(methoxy)ethoxymethoxy)-3-trityloxymethyl)cyclopentyloxy]tert-butyldimethylsilaneC42H54O6SiEe = 100%[α]D = +28.1 (c 0.26, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1S,2S,3S,4S)
(1R,3S,4S)-[(4-(2-(Methoxy)ethoxymethoxy)-3-trityloxymethyl)cyclopentyloxy]tert-butyldimethylsilaneC35H48O5SiEe = 100%[α]D = −6.7 (c 0.90, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,3S,4S)
(1R,2S,3R,4S)-[2-Acetoxymethyl-3-benzyloxy-4-(tert-butyldimethylsilyloxy)]cyclopent-1-yl acetateC26H36O6SiEe = 100%[α]D = +61.6 (c 0.45, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3R,4S)
(1R,2S,3S,4S)-[2-Acetoxymethyl-3-benzyloxy-4-(tert-butyldimethylsilyloxy)]cyclopent-1-yl acetateC26H36O6SiEe = 100%[α]D = −41.3 (c 0.6, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3S,4S)
(1R,2S,4R)-[2-Acetoxymethyl-4-(tert-butyldimethylsilyloxy)]cyclopent-1-yl acetateC16H30O5SiEe = 100%[α]D = −42.6 (c 0.46, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,4R)
(1S,2R,3S,4S)-[2-Benzyloxy-4-(2-(methoxy)ethoxymethoxy)-3-trityloxymethyl]cyclopentanolC36H40O6Ee = 100%[α]D = +16.5 (c 0.65, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1S,2R,3S,4S)
(1S,2S,3S,4S)-[2-Benzyloxy-4-(2-(methoxy)ethoxymethoxy)-3-trityloxymethyl]cyclopentanolC36H40O6Ee = 100%[α]D = +16.9 (c 0.54, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1S,2S,3S,4S)
(1R,3S,4S)-[4-(2-(Methoxy)ethoxymethoxy)-3-trityloxymethyl]cyclopentanolC29H34O5Ee = 100%[α]D = +2.9 (c 0.60, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,3S,4S)
(1R,2S,3R,4S)-[2-Acetoxymethyl-3-benzyloxy-4-hydroxy]cyclopent-1-yl acetateC17H22O6Ee = 100%[α]D = +23.6 (c 0.55, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3R,4S)
(1R,2S,3S,4S)-[2-Acetoxymethyl-3-benzyloxy-4-hydroxy]cyclopent-1-yl acetateC17H22O6Ee = 100%[α]D = −55.7 (c 0.45, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,3S,4S)
(1R,2S,4R)-[2-Acetoxymethyl-4-hydroxy]cyclopent-1-yl acetateC10H16O5Ee = 100%[α]D = −40.0 (c 0.34, CHCl3)Source of chirality: chiral starting materialAbsolute configuration: (1R,2S,4R)
Journal: Tetrahedron: Asymmetry - Volume 20, Issues 6–8, 7 May 2009, Pages 821–831