| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1349860 | 1500371 | 2009 | 8 صفحه PDF | دانلود رایگان |
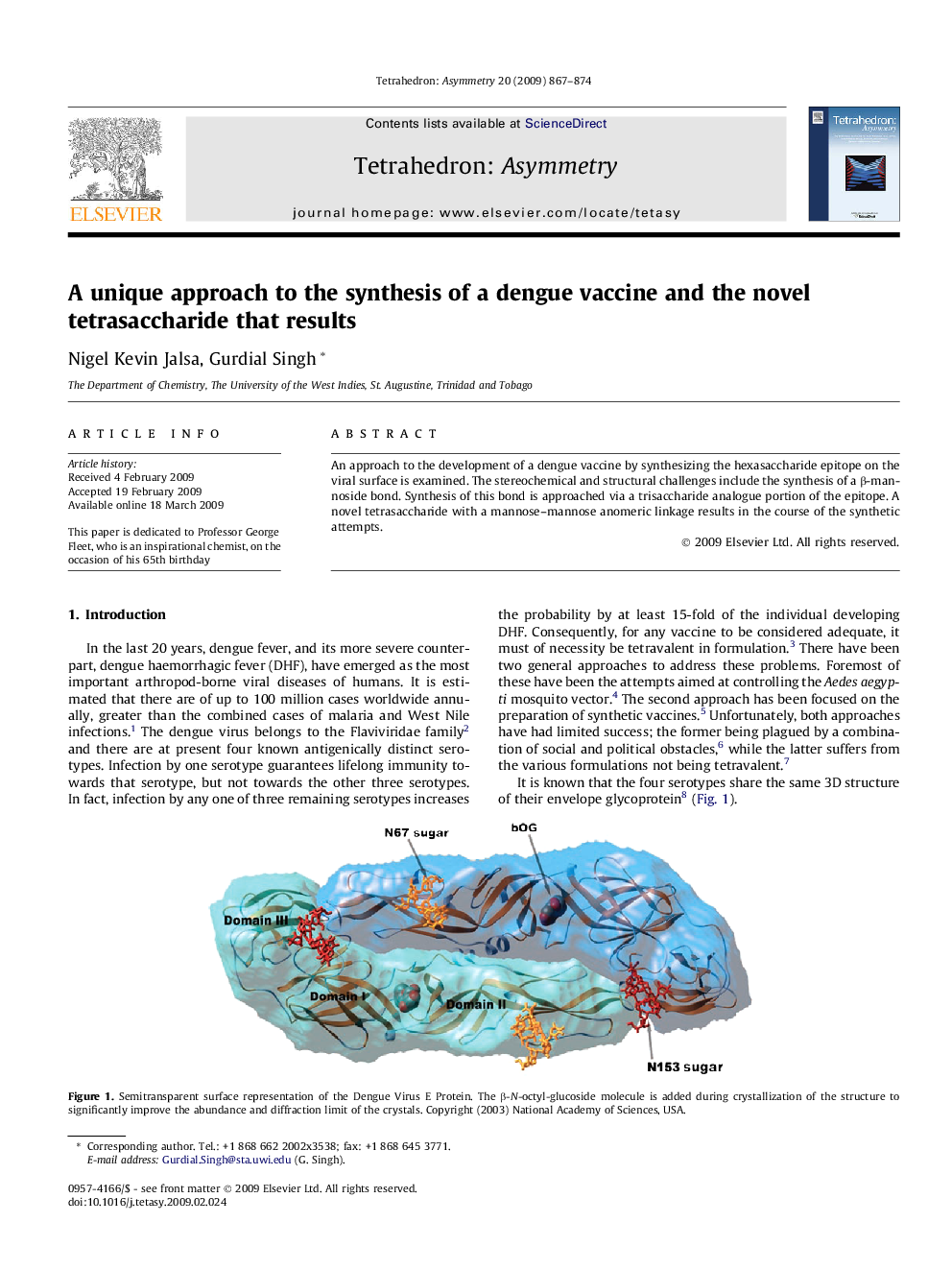
An approach to the development of a dengue vaccine by synthesizing the hexasaccharide epitope on the viral surface is examined. The stereochemical and structural challenges include the synthesis of a β-mannoside bond. Synthesis of this bond is approached via a trisaccharide analogue portion of the epitope. A novel tetrasaccharide with a mannose–mannose anomeric linkage results in the course of the synthetic attempts.
Figure optionsDownload as PowerPoint slide
2,3,4,6-Tetra-O-acetyl-α-d-mannopyranosyl-1-O-propane-1,3-diyl phosphateC17H25O13P[α]D24=+33.5 (c 1.0, CHCl3)Source of chirality: d-mannose
2,3-Di-O-benzyl-α,β-d-mannopyranoseC20H24O6[α]D24=-27.2 (c 1.03, CHCl3)Source of chirality: methyl-α-d-mannopyranoside
2′,3′,4′,6′-Tetra-O-acetyl-α-d-mannopyranosyl-(1′→6)-(2″,3″,4″,6″-tetra-O-α-d-acetyl-mannopyranosyl-(1″→4))-(2′′′,3′′′,4′′′,6′′′-tetra-O-acetyl-α-d-mannopyranosyl-(1′′′→1))-2,3-di-O-benzyl-α-d-mannopyranosideC62H78O33[α]D24=+42.2 (c, 10.6, CHCl3)Source of chirality: d-mannose
4,6-O-Benzylidene-1,2,3-tri-O-benzyl-β-d-mannopyranosideC34H34O6[α]D24=-14.3 (c 0.5, CHCl3)Source of Chirality: d-mannose
1,2,3-Tri-O-benzyl-β-d-mannopyranosideC27H30O6[α]D24=-29.9 (c 0.34, CHCl3)Source of chirality: d-mannose
2′,3′,4′,6′-Tetra-O-acetyl-α-d-mannopyranosyl-(1′→6)-(2″,3″,4″,6″-tetra-O-α-d-acetyl-mannopyranosyl-(1″→4))-1,2,3-tri-O-benzyl-α-d-mannopyranosideC55H66O24[α]D24=+8.9 (c 2.1, CHCl3)Source of chirality: d-mannose
2′,3′,4′,6′-Tetra-O-acetyl-α-d-mannopyranosyl-(1′→6)-(2″,3″,4″,6″-tetra-O-α-d-acetyl-mannopyranosyl-(1″→4))-2,3-tri-O-benzyl-α-d-mannopyranoseC48H60O24[α]D24=+95.2 (c, 0.01, CHCl3)Source of chirality: d-mannose
Journal: Tetrahedron: Asymmetry - Volume 20, Issues 6–8, 7 May 2009, Pages 867–874