| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1350170 | 980426 | 2008 | 7 صفحه PDF | دانلود رایگان |
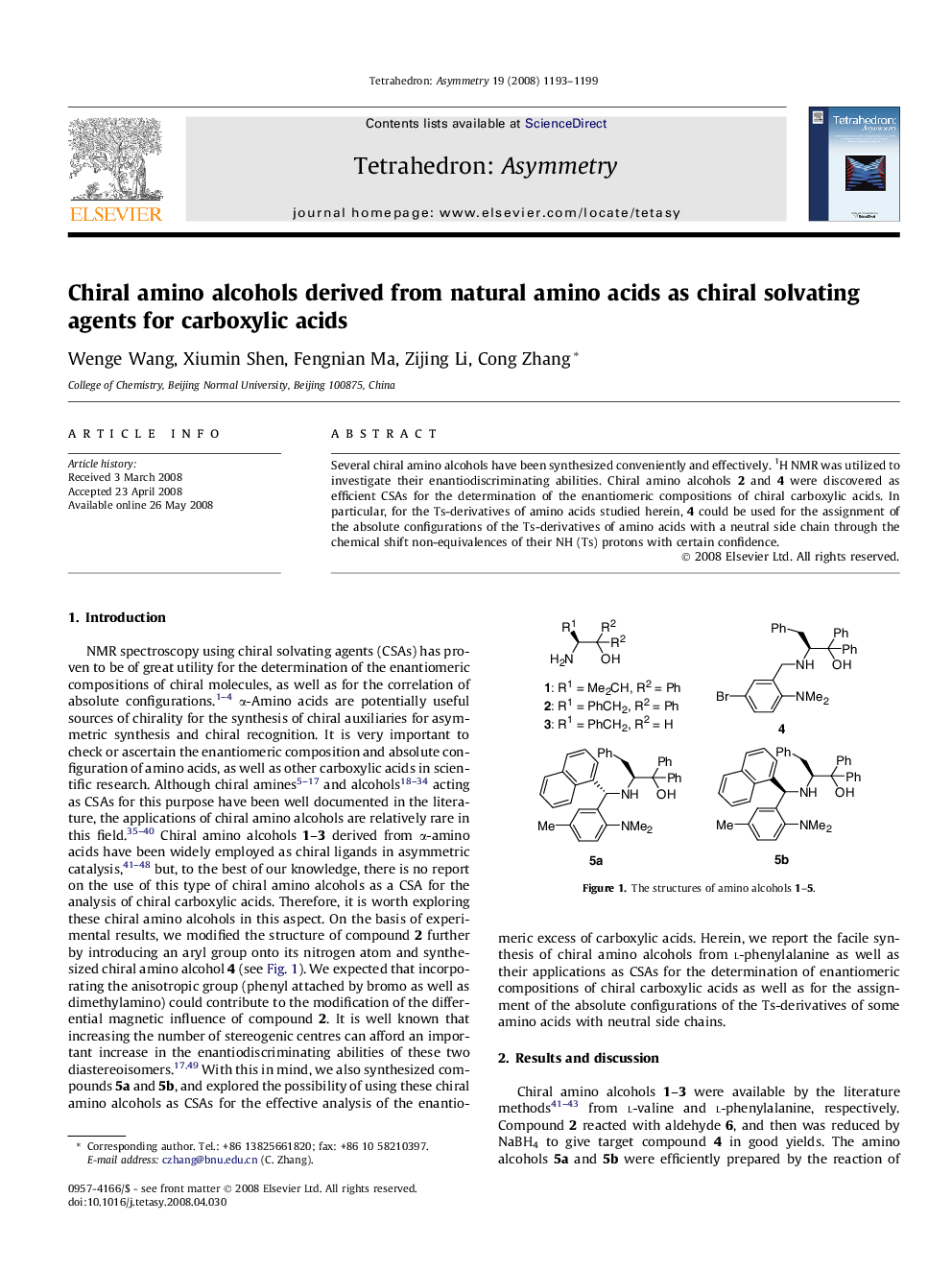
Several chiral amino alcohols have been synthesized conveniently and effectively. 1H NMR was utilized to investigate their enantiodiscriminating abilities. Chiral amino alcohols 2 and 4 were discovered as efficient CSAs for the determination of the enantiomeric compositions of chiral carboxylic acids. In particular, for the Ts-derivatives of amino acids studied herein, 4 could be used for the assignment of the absolute configurations of the Ts-derivatives of amino acids with a neutral side chain through the chemical shift non-equivalences of their NH (Ts) protons with certain confidence.
Figure optionsDownload as PowerPoint slide
(S)-2-(5-Bromo-2-dimethylamino-benzylamino)-1,1,3-triphenyl-propan1-1-olC30H31N2OBr[α]D20=-41.6 (c 0.44, CHCl3)Source of chirality: l-phenylalanineAbsolute configuration: (S)
(S,S)-2-{[(2-Dimethylamino-5-methylphenyl)-naphthalen-1-ylmethyl]amino}-1,1,3-triphenyl-propan-1-olC41H40N2O[α]D20=+42.8 (c 0.50, CHCl3)Source of chirality: l-phenylalanineAbsolute configuration: (S,S)
(S,R)-2-{[(2-Dimethylamino-5-methylphenyl)-naphthalen-1-ylmethyl]amino}-1,1,3-triphenyl-propan-1-olC41H40N2O[α]D20=-34.9 (c 0.47, CHCl3)Source of chirality: l-phenylalanineAbsolute configuration: (S,R)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 10, 30 May 2008, Pages 1193–1199