| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1350265 | 980433 | 2008 | 6 صفحه PDF | دانلود رایگان |
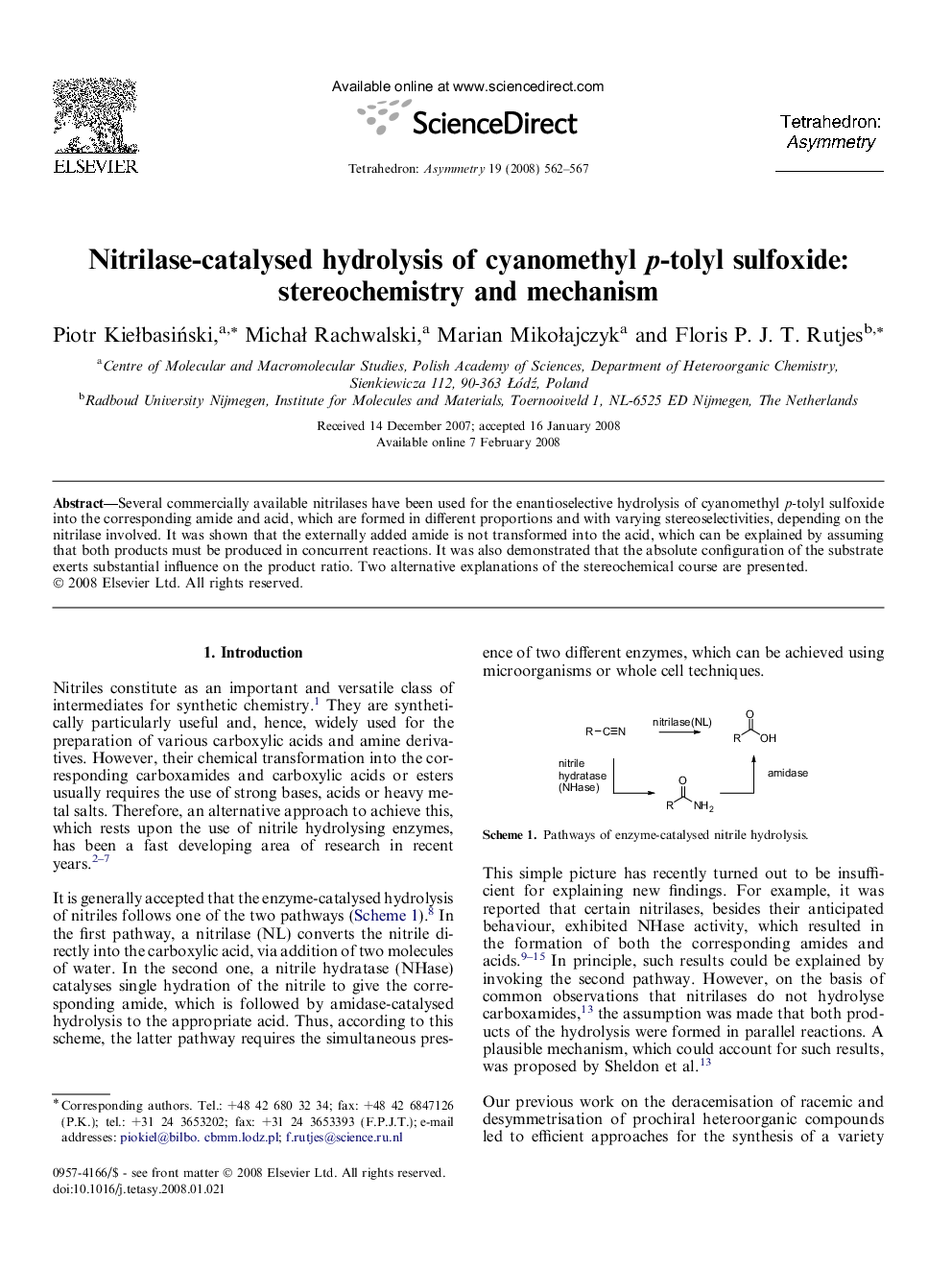
Several commercially available nitrilases have been used for the enantioselective hydrolysis of cyanomethyl p-tolyl sulfoxide into the corresponding amide and acid, which are formed in different proportions and with varying stereoselectivities, depending on the nitrilase involved. It was shown that the externally added amide is not transformed into the acid, which can be explained by assuming that both products must be produced in concurrent reactions. It was also demonstrated that the absolute configuration of the substrate exerts substantial influence on the product ratio. Two alternative explanations of the stereochemical course are presented.
Figure optionsDownload as PowerPoint slide
p-ToluenesulfinylacetamideC9H11NO2SEe = 95%[α]D = −230.0 (c 1, MeOH)Source of chirality: enzymatic kinetic resolutionAbsolute configuration: (S) (comparative CD analysis and chemical correlation)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 5, 18 March 2008, Pages 562–567