| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1350275 | 980433 | 2008 | 4 صفحه PDF | دانلود رایگان |
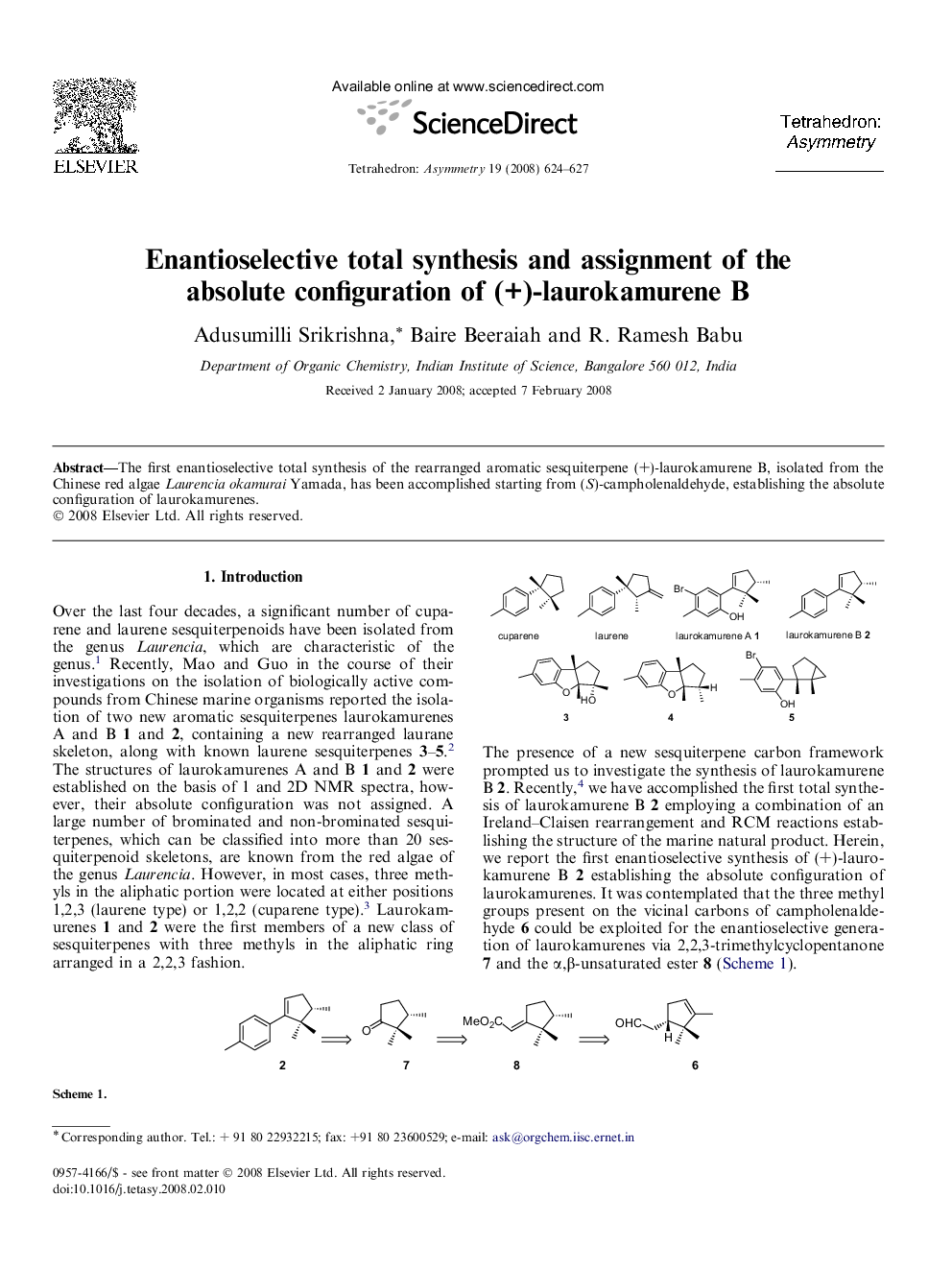
The first enantioselective total synthesis of the rearranged aromatic sesquiterpene (+)-laurokamurene B, isolated from the Chinese red algae Laurencia okamurai Yamada, has been accomplished starting from (S)-campholenaldehyde, establishing the absolute configuration of laurokamurenes.
Figure optionsDownload as PowerPoint slide
Methyl 2-[(1S,3S)-2,2,3-trimethylcyclopent-1-yl]acetateC11H20O2[α]D23=-9.0 (c 3.8, CHCl3)Source of chirality: campholenaldehydeAbsolute configuration: (1S,3S)
Methyl 2-[(3S)-2,2,3-trimethylcyclopent-1-ylidene]acetateC11H28O2[α]D24=-7.5 (c 4.6, CHCl3)Source of chirality: campholenaldehydeAbsolute configuration: (3S)
(1R,3S)1-[4-Methylphenyl]-2,2,3-trimethylcyclopentan-1-olC15H22O[α]D23=+10.7 (c 1.4, CHCl3)Source of chirality: campholenaldehydeAbsolute configuration: (1R,3S)
Journal: Tetrahedron: Asymmetry - Volume 19, Issue 5, 18 March 2008, Pages 624–627