| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1350726 | 980462 | 2006 | 6 صفحه PDF | دانلود رایگان |
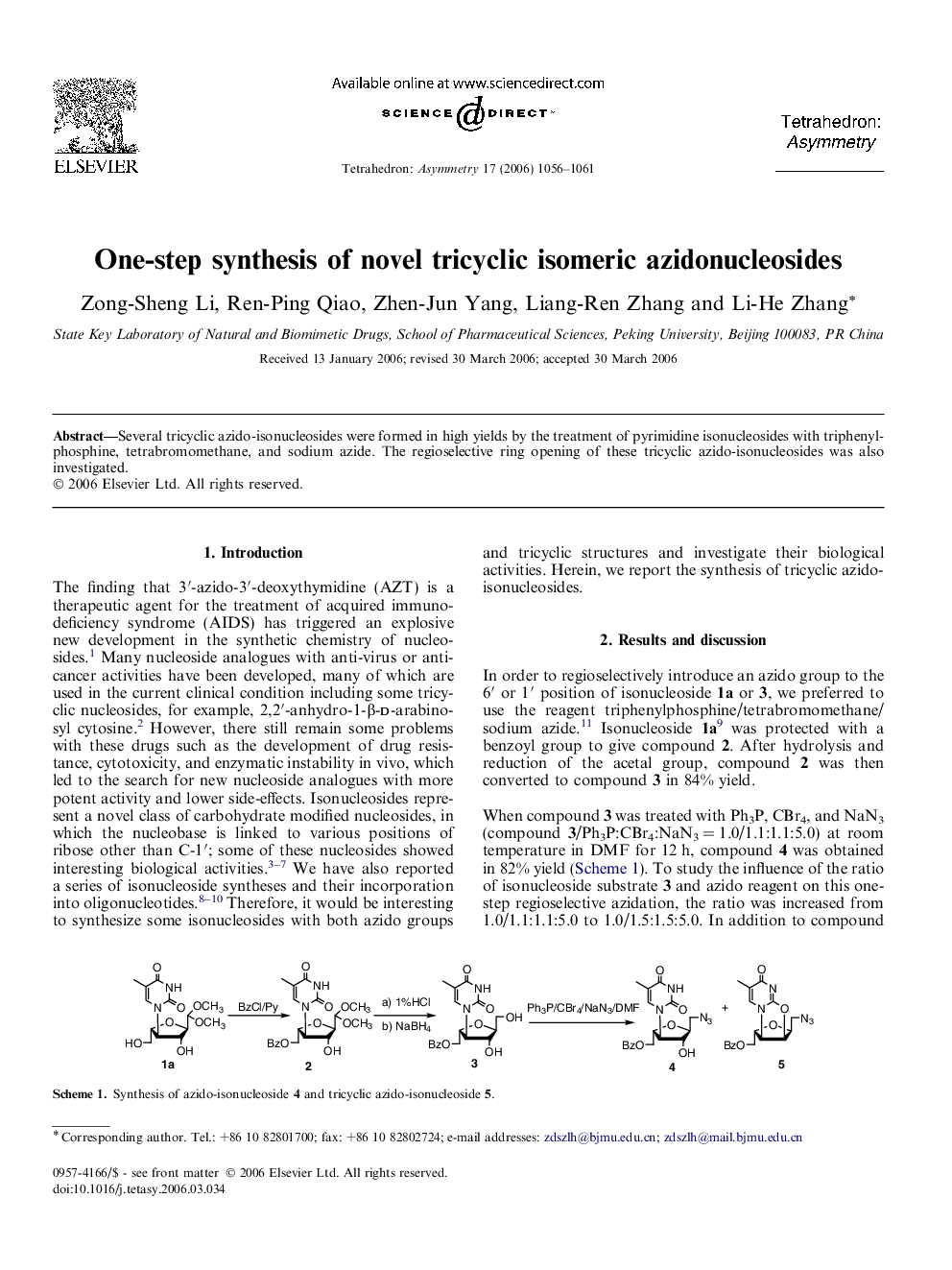
Several tricyclic azido-isonucleosides were formed in high yields by the treatment of pyrimidine isonucleosides with triphenylphosphine, tetrabromomethane, and sodium azide. The regioselective ring opening of these tricyclic azido-isonucleosides was also investigated.
Figure optionsDownload as PowerPoint slide
6′-O-Benzoyl-1′-deoxy-1′-azido-4′-deoxy-4′-(thymin-1-yl)-2′,5′-anhydro-l-mannitolC18H19N5O6[α]D24=-39.2 (c 0.120, MeOH)Source of chirality: l-mannitol
6′-O-Benzoyl-1′-deoxy-1′-azido-4′-deoxy-4′-(thymin-1-yl)-2,3′:2′,5′-dianhydro-l-altritolC18H17N5O5[α]D24=+28.9 (c 0.127, MeOH)Source of chirality: l-altriol
6′-Deoxy-6′-azido-4′-deoxy-4′-(thymin-1-yl)-2′,5′-anhydro-l-mannofuranose dimethyl acetalC13H19N5O6[α]D24=-19.6 (c 0.160, MeOH)Source of chirality: l-mannofuranose
6′-Deoxy-6′-azido-4′-deoxy-4′-(uracil-1-yl)-2′,5′-anhydro-l-mannofuranose dimethyl acetalC12H17N5O6[α]D24=-23.8 (c 0.080, MeOH)Source of chirality: l-mannofuranose
6′-Deoxy-6′-azido-4′-deoxy-4′-(thymin-1-yl)-2,3′:2′,5′-dianhydro-l-altrofuranose dimethyl acetalC13H17N5O5[α]D24=+79.4 (c 0.152, MeOH)Source of chirality: l-altrofuranose
6′-Deoxy-6′-azido-4′-deoxy-4′-(uracil-1-yl)-2,3′:2′,5′-dianhydro-l-altrofuranose dimethyl acetalC12H15N5O5[α]D24=+92.7 (c 0.070, MeOH)Source of chirality: l-altrofuranose
1′- Deoxy-1′-azido-4′-deoxy-4′-(thymin-1-yl)-2,3′:2′,5′-dianhydro-l-altritolC11H13N5O4[α]D24=+45.0 (c 0.030, MeOH)Source of chirality: l-altriol
1′-Deoxy-1′-azido-4′-deoxy-4′-(5-methyl-N2-methyl-iso-cytosin-1-yl)-2′,5′-anhydro-l-altritolC12H18N6O4[α]D24=-82.3 (c 0.038, MeOH)Source of chirality: l-altriol
1′-Deoxy-1′-azido-4′-deoxy-4′-(thymin-1-yl)-2′,5′-anhydro-l-altritolC11H15N5O5[α]D24=-38.5 (c 0.040, MeOH)Source of chirality: l-altriol
1′-Deoxy-1′-amino-4′-deoxy-4′-(thymin-1-yl)-2,1′:2′,5′-dianhydro-l-altritolC11H15N3O4[α]D24=+40.8 (c 0.105, MeOH)Source of chirality: l-altriol
Journal: Tetrahedron: Asymmetry - Volume 17, Issue 7, 3 April 2006, Pages 1056–1061